A) 5.0 × 10-1 kJ
B) 1.2 kJ
C) 5.0 × 102 kJ
D) 1.2 × 103 kJ
E) 1.6 × 103 kJ
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is incorrectly matched?
A) Radiant energy; solar energy able to influence global climate patterns
B) Thermal energy; related to temperature irrespective of the volume
C) Energy; capacity to do work
D) Chemical energy; potential energy
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Natural gas,or methane,is an important fuel.Combustion of one mole of methane releases 802.3 kJ of energy.How much energy does that represent in kcal? (1 cal = 4.184 J)
A) 1.92 × 10-1 kcal
B) 1.92 × 102 kcal
C) 3.36 × 103 kcal
D) 1.92 × 105 kcal
E) 3.36 × 106 kcal
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose a 0.500-g sample of an organic compound is analyzed via bomb calorimetry.The temperature of the calorimeter is measured over time.At t = 5 min,the combustion reaction is initiated.Below is a plot of the data that are obtained. 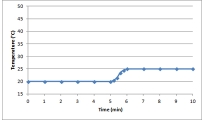 Suppose the experiment is repeated under identical conditions,but with a 1.000-g sample of the organic compound.What might a plot of the resulting data look like?
Suppose the experiment is repeated under identical conditions,but with a 1.000-g sample of the organic compound.What might a plot of the resulting data look like?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A system which does work on the surroundings with no heat change has
A) w < 0,ΔU = 0.
B) W > 0,ΔU > 0.
C) W > 0,ΔU < 0.
D) W < 0,ΔU > 0.
E) W < 0,ΔU < 0.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A system contracts from an initial volume of 15.0 L to a final volume of 10.0 L under a constant external pressure of 0.80 atm.What is W? (1 L·atm = 101.3 J)
A) -4.0 J
B) +4.0 J
C) -4.1 ×102 J
D) +4.1 ×102 J
E) +81 J
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heat of solution of ammonium nitrate is 26.2 kJ/mol.If a 5.368 g sample of NH4NO3 is added to 40.0 mL of water in a calorimeter at 23.5°C,what is the final temperature of the solution? The specific heat of water is 4.18 J/g·°C and the heat capacity of the calorimeter is 0.650 kJ/°C.
A) 14.3°C
B) 20.8°C
C) -7.7°C
D) 25.6°C
E) 21.4°C
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which represents an enthalpy change at 25 °C and 1 atm that is equal to ΔHof for H2O(l) ?
A) O2(g) + 2H2(g) → 2H2O(l)
B) ½O2(g) + H (g) → H2O(g)
C) H2(g) + ½O2(g) + → H2O(l)
D) 2H2O(l) → O2(g) + 2H2(g)
E) H2O(l) → ½O2(g) + H2(g)
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A piece of copper metal is initially at 100.0°C.It is dropped into a coffee cup calorimeter containing 50.0 g of water at a temperature of 20.0°C.After stirring,the final temperature of both copper and water is 25.0°C.Assuming no heat losses,and that the specific heat (capacity) of water is 4.18 J/g·°C,what is the heat capacity of the copper in J/°C?
A) 2.79 J/°C
B) 3.33 J/°C
C) 13.9 J/°C
D) 209 J/°C
E) None of these choices is correct.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When Karl Kaveman adds chilled grog to his new granite mug,he removes 10.9 kJ of energy from the mug.If it has a mass of 625 g and was at 25°C,what is its new temperature? Specific heat capacity of granite = 0.79 J/g·°C.
A) 3°C
B) 14°C
C) 22°C
D) 47°C
E) None of these choices is correct.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ethanol,C2H5OH,is promoted as a clean fuel and is used as an additive in many gasoline mixtures.Calculate the ΔH°rxn for the combustion of ethanol. Substance ΔH°f(kJ/mol) C2H5OH(l) -277.0 CO2(g) -393.5 H2O(g) -241.8
A) -1235.4 kJ
B) -751.8 kJ
C) -358.3 kJ
D) 358.3 kJ
E) 1235.4 kJ
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the standard enthalpy of formation of liquid methanol,CH3OH(l) ? C(graphite) + O2(g) → CO2(g) ΔH°rxn = -393.5 kJ/mol H2(g) + ½O2→ H2O(l) ΔH°rxn = -285.8 kJ/mol CH3OH(l) + 3/2O2(g) → CO2(g) + 2H2O(l) ΔH°rxn = -726.4 kJ/mol
A) -1691.5 kJ/mol
B) -238.7 kJ/mol
C) 1691.5 kJ/mol
D) 47.1 kJ/mol
E) -47.1 kJ/mol
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that CaO(s) + H2O(l) → Ca(OH) 2(s) ,ΔH°rxn = -64.8 kJ/mol,how many grams of CaO must react in order to liberate 525 kJ of heat?
A) 6.92 g
B) 56.1 g
C) 454 g
D) 606 g
E) 3.40 × 104 g
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using Hess' law,what is ΔH°rxn at 25°C for the following reaction? ClF(g) + F2(g) → ClF3(g) 2ClF(g) + O2(g) → Cl2O(g) + OF2(g) ΔH°rxn = +167.4kJ/mol 2ClF3(g) + 2O2(g) → Cl2O(g) +3OF2(g) ΔH°rxn = +341.4kJ/mol 2F2(g) + O2(g) → 2OF2(g) ΔH°rxn = -43.4 kJ/mol
A) -217.5 kJ/mol
B) -130.2 kJ/mol
C) 217.5 kJ/mol
D) -108.7 kJ/mol
E) 465.4 kJ/mol
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 1.00-g sample of octane (C8H18) is burned in a bomb calorimeter that has a heat capacity of 5.80 kJ/°C.The temperature of the calorimeter rises from 25.00°C to 33.20°C.What is ΔU per mole for the combustion of octane?
A) -47.6 kJ/mol
B) -416 kJ/mol
C) -707 kJ/mol
D) -5.43 ×103 kJ/mol
E) -1.86 ×105 kJ/mol
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A system that does no work but which transfers heat to the surroundings has
A) q < 0,ΔU > 0.
B) q < 0,ΔU < 0.
C) q > 0,ΔU > 0.
D) q > 0,ΔU < 0.
E) q < 0,ΔU = 0.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Pentaborane B5H9(s) burns vigorously in O2 to give B2O3(s) and H2O(l) .What is ΔH° for the combustion of 1 mol of B5H9(s) ? Substance ΔH°f (kJ/mol) B2O3(s) -1273.5 B5H9(s) +73.2 H2O(l) -285.8
A) -1486.1 kJ
B) -1632.5 kJ
C) -4396.7 kJ
D) -4652.85 kJ
E) -9086.1 kJ
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The enthalpy of vaporization of a compound is always positive.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much heat is released if 35.0 g of ethanol (C2H5OH) burns in excess oxygen? C2H5OH(l) + 3O2(g) → 2 CO2(g) + 3 H2O(l) ΔH°rxn = -1367 kJ/mol
A) 1797 kJ
B) 1367 kJ
C) 9.61 × 10-4 kJ
D) 4.78 × 104 kJ
E) 1040 kJ
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
ΔH does not depend on the path of a reaction,but ΔU does.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 134
Related Exams