A) N2O
B) CS2
C) PH3
D) CCl4
E) NO2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an element is bonded to 4 other atoms and has a formal charge of +1,what group must the element be in?
A) 3A
B) 4A
C) 5A
D) 6A
E) 7A
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these Lewis structures is incorrect?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ignoring resonance,the formal charge on the nitrogen atom in the nitrate ion is
A) +2.
B) +1.
C) 0.
D) -1.
E) -2.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the element whose Lewis symbol is correct.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
_____________________ of atoms interact to form compounds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s) .What does the symbol represent?
A) Electron configuration
B) Valence electrons
C) Atomic number
D) Atomic mass
E) Nucleus and core electrons
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In Ba(CN) 2,the bonding is
A) essentially ionic.
B) essentially covalent.
C) both ionic and covalent.
D) neither ionic nor covalent.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Lewis theorized the octet rule to describe chemical bonding where atoms lose,gain,or share electrons in order to achieve a noble gas configuration.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these compounds is most likely to be ionic?
A) NCl3
B) BaCl2
C) CO
D) SO2
E) SF4
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these substances will display an incomplete octet in its Lewis structure?
A) CO2
B) Cl2
C) ICl
D) NO
E) SO2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis dot symbol for the calcium ion is
A) ![]() 2+
2+
B) ![]()
C) ![]() 2+
2+
D) Ca2+
E) Ca
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following contains covalent bonds?
A) BaO
B) IBr
C) Mg
D) LiBr
E) Cu
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of Lewis structures is not a pair of resonance structures?
A) ![]()
B) ![]()
C) ![]()
D) All of these pairs are resonance structures of the same species.
E) None of these pairs is resonance structures of the same species.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these elements is most likely to exhibit an expanded octet in its compounds?
A) O
B) S
C) Na
D) C
E) N
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Ionic compounds tend to form between metals and nonmetals when electrons are transferred from an element with high ionization energy (metal)to an element with a low electron affinity (nonmetal).
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
A(n)_____________ is a representation of covalent bonding in which shared electron pairs are shown either as dashes or as pairs of dots between two atoms and unshared electrons are shown as dots around the individual atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrazine,N2H4,is a good reducing agent that has been used as a component in rocket fuels. Select its Lewis structure.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of the choices is correct.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these molecules has an atom with an expanded octet?
A) HCl
B) AsCl5
C) ICl
D) NCl3
E) Cl2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many lone pairs of electrons need to be added to complete this Lewis structure? 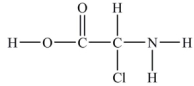
A) 5
B) 8
C) 6
D) 1
E) 16
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 95
Related Exams