Correct Answer

verified
Correct Answer
verified
Essay
Draw a correct Lewis structure for boric acid, B(OH)3.
Correct Answer

verified
11eab45f_ce66_6054_acdb_fbc1085a3047_TB6198_00 or 11eab45f_ce66_6055_acdb_6fde8f62a997_TB6198_00
Correct Answer
verified
Essay
Draw the line energy orbital diagram for the outer shell of an uncharge nitrogen atom and describe the location and number of unshared electrons.
Correct Answer

verified
 There are three unshared elec...
There are three unshared elec...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Nitroamines are common functional groups found in energetic materials, such as RDX and HMX. For the structure below, draw two other significant resonance structures, include any formal charges, and indicate the hybridization on each nitrogen and oxygen. 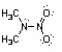
Correct Answer

verified
All nitrog...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Provide the line-angle formula (skeletal structure) for (CH3CH2)2C=O.
Correct Answer

verified
Correct Answer
verified
Essay
Provide the products of the following acid-base reaction. (CH3)3NH+ + HO-→
Correct Answer

verified
Correct Answer
verified
Essay
Draw a correct Lewis structure for (CH3)2CHCOOH.
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the molecular formula for the molecule shown? 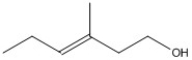
Correct Answer

verified
Correct Answer
verified
Essay
Write a completed equation for the acid-base pair shown below. HCO2H + -NH2 →
Correct Answer

verified
HCO2H + -NH2 ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Provide the line-angle formula (skeletal structure) for CH2=CHCH2CH2C(CH3)3.
Correct Answer

verified
Correct Answer
verified
Essay
Which is more acidic, HF or HI? Explain.
Correct Answer

verified
HI is more acidic. As a conjug...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw the important resonance forms for the structure shown below. 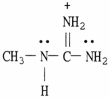
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which sequence correctly ranks the following protons in order of increasing pKa value? 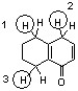
A) 3<1<2
B) 2<1<3
C) 3<2<1
D) 1<3<2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
The compound phenol is shown below. Provide the structure of the conjugate base of phenol. 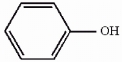
Correct Answer

verified
Correct Answer
verified
Short Answer
For most compounds in which a nitrogen atom bears no formal charge, the valence of this nitrogen atom is ________.
Correct Answer

verified
3
Correct Answer
verified
Multiple Choice
The pH of a 150 mL aqueous solution of 2.13 x 10-3 M HCl is ________.
A) -3.000
B) 3.000
C) 2.672
D) 2.130
E) none of the above
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw a correct Lewis structure for acetaldehyde, CH3CHO.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules contains a polar covalent bond?
A) H2
B) F2
C) CH3Cl
D) NaCl
E) He
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Write a completed equation for the acid-base pair shown below. HCN + NaOH →
Correct Answer

verified
HCN + NaOH → NaCN + H2O
Correct Answer
verified
Essay
Provide the line-angle formula for CH3CH2C(CH3)2CH2CHO
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 127
Related Exams