Correct Answer

verified
Correct Answer
verified
Multiple Choice
The lone pairs on oxygen in the following compound are _______. 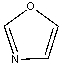
A) both localized
B) both delocalized
C) one localized
D) one delocalized
E) Both C & D
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many total lone pairs of electrons are on both oxygen atoms in the following compound? 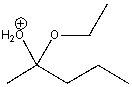
A) one
B) two
C) three
D) four
E) none
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct bond-line structure for (CH3) 2CHCH2CH3? 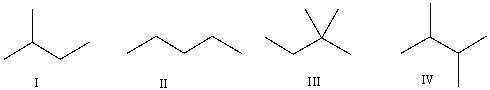
A) I
B) II
C) III
D) IV
E) None of these
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds contain a ketone functional group? 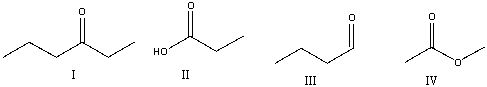
A) I
B) II
C) III
D) IV
E) All of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds contain an alcohol functional group? 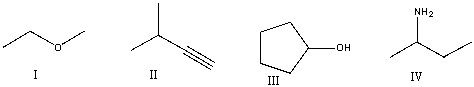
A) I
B) II
C) III
D) IV
E) None of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct Lewis structure for CH3(CH2) 2OH? 
A) I
B) II
C) III
D) IV
E) Both II & III
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The lone pair on nitrogen in the following compound is _______. 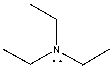
A) localized
B) delocalized
D) undefined
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a correct resonance structure for compound A? 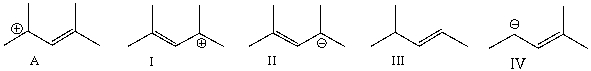
A) I
B) II
C) III
D) IV
E) None of these
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following transformation how many H atoms are added or lost? 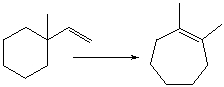
A) Added one
B) Added two
C) Lost one
D) Lost two
E) No change
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following violates the rules for curved arrows? 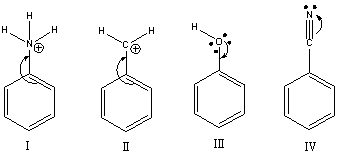
A) I
B) II & IV
C) I & III
D) III & IV
E) None of these
G) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Essay
For the following compound identify the lone pairs and indicate if each lone pair is localized or delocalized. 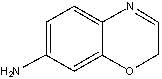
Correct Answer

verified
Correct Answer
verified
Essay
Draw the curved arrow(s) for converting the first resonance structure into the second resonance structure. 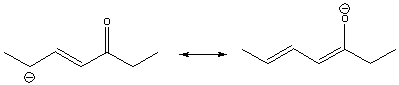
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the relationship between the following compounds? 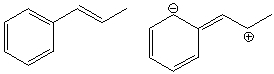
A) Constitutional isomers
B) Resonance structures
C) conformers
D) Identical compounds
E) Different compounds
G) D) and E)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following is the correct bond-line structure for (CH3) 4C? 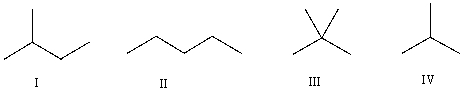
A) I
B) II
C) III
D) IV
E) None of these
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
For the following compound identify the lone pairs and indicate if each lone pair is localized or delocalized. 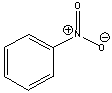
Correct Answer

verified
Correct Answer
verified
Essay
Explain using words as well as structural drawings, if the single curved arrow shown is sufficient to draw the resonance structure. 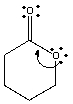
Correct Answer

verified
The single arrow shown will violate the octet rule. Drawing another curved arrow will remove the violation. 11eab459_a72e_ea87_acdb_5d9e7fb042ad_TB3185_00
Correct Answer
verified
Essay
Draw a bond-line structure for CH3CH2O(CH2)2CH(CH3)2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge on the nitrogen atom in the following compound? 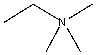
A) -1
B) -2
C) +1
D) +2
E) 0
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds contain an amine functional group? 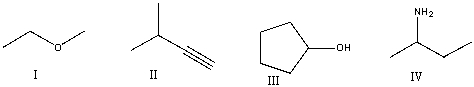
A) I
B) II
C) III
D) IV
E) None of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 154
Related Exams