A) carbonyl
B) ketone
C) aldehyde
D) carboxyl
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
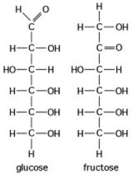 - The figure shows the structures of glucose and fructose. These two molecules are ________.
- The figure shows the structures of glucose and fructose. These two molecules are ________.
A) isotopes
B) enantiomers
C) cis-trans isomers
D) structural isomers
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A.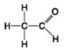 B.
B.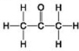 C.
C.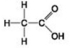 D.
D.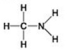 - Which molecule shown has a carbonyl functional group in the form of an aldehyde?
- Which molecule shown has a carbonyl functional group in the form of an aldehyde?
A) A
B) B
C) C
D) D
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A. B.
B. C.
C. D.
D. - Which molecule shown contains a carboxyl group?
- Which molecule shown contains a carboxyl group?
A) A
B) B
C) C
D) D
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A. B.
B. C.
C. D.
D. -Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
-Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
A) A
B) B
C) C
D) D
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
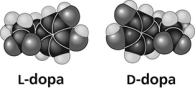 Thalidomide and L-dopa (see figure) are examples of pharmaceutical drugs that occur as enantiomers, or molecules that ________.
Thalidomide and L-dopa (see figure) are examples of pharmaceutical drugs that occur as enantiomers, or molecules that ________.
A) have identical three-dimensional shapes
B) are mirror images of one another
C) are mirror images of one another and have the same biological activity
D) are cis-trans isomers
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is carbon so important in biology?
A) It is a common element on Earth.
B) It has very little electronegativity, making it a good electron donor.
C) It bonds to only a few other elements.
D) It can form a variety of carbon skeletons and host functional groups.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A.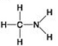 B.
B.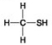 C.
C.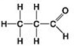 D.
D.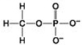 - Which molecule shown above can contribute negative charge when positioned in a chain?
- Which molecule shown above can contribute negative charge when positioned in a chain?
A) A
B) B
C) C
D) D
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound contains hydroxyl groups as its predominant functional group. Therefore, this compound ________.
A) lacks an asymmetric carbon and is probably a fat or lipid
B) should dissolve in water
C) should dissolve in a nonpolar solvent
D) will not form hydrogen bonds with water
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other? Testosterone and estradiol ________.
A) are structural isomers but have the same molecular formula
B) are cis-trans isomers but have the same molecular formula
C) have different functional groups attached to the same carbon skeleton
D) are enantiomers of the same organic molecule
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following carbon molecules does not have the bond angle of 109.5°?
A) CH₄
B) C2H4
C) C2H6
D) C3H8
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why are hydrocarbons insoluble in water?
A) The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B) The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
C) They exhibit considerable molecular complexity and diversity.
D) They are less dense than water.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
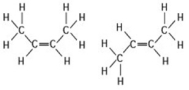 The two molecules shown in the figure are best described as ________.
The two molecules shown in the figure are best described as ________.
A) enantiomers
B) radioactive isotopes
C) structural isomers
D) cis-trans isomers
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Visualise the structural formula of each of the following hydrocarbons. Which hydrocarbon has a double bond in its carbon skeleton?
A) C₃H₈
B) C₂H₆
C) C₂H₄
D) C₂H₂
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Compared to a hydrocarbon chain where all the carbon atoms are linked by single bonds, a hydrocarbon chain with the same number of carbon atoms but with one or more double bonds will ________.
A) be more flexible in structure
B) be more constrained in structure
C) be more polar
D) have more hydrogen atoms
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Organic chemistry is currently defined as
A) the study of compounds made only by living cells.
B) the study of carbon compounds.
C) the study of natural (as opposed to synthetic) compounds.
D) the study of hydrocarbons.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons does one atom of carbon share to complete its valence shell?
A) 2
B) 3
C) 4
D) 8
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbon atom has 6 electrons however, its valency is 4. This is because the carbon atom ________.
A) donates its 2 electrons to another atom
B) shares its 2 electrons and bonds with another atom
C) has 4 electrons in its first shell and 2 in the second shell
D) has only 2 electrons in its first shell and 4 in the second shell
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A. B.
B. C.
C. D.
D. - Which molecule shown is a thiol?
- Which molecule shown is a thiol?
A) A
B) B
C) C
D) D
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describes cis-trans isomers?
A) They have variations in arrangement around a double bond.
B) They have an asymmetric carbon that makes them mirror images.
C) They have the same chemical properties.
D) They have different molecular formulas.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 58
Related Exams