A) pentane, C5H12 , CH3CH2CH2CH2CH3
B) hexane, C6H14 , CH3CH2CH2CH2CH2CH3
C) isooctane, C8H18 , (CH3) 3CCH2CH(CH3) 2
D) heptane, C7H16 , CH3CH2CH2CH2CH2CH2CH3
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At current usage rates, world reserves of which fuel below are estimated to last for the least number of years?
A) natural gas
B) hydrogen
C) petroleum
D) coal
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fermentation of carbohydrates is a source of
A) MTBE.
B) ethanol.
C) ethers.
D) methanol.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complete combustion of C2H6 yields
A) C2H4 and H2.
B) CO and H2O.
C) CO2 and H2O.
D) C and H2.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hydrocarbon represented by the following model would be called 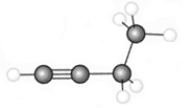
A) unsaturated.
B) aromatic.
C) an alkene.
D) all of the above
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which fossil fuel has a molecular structure containing cyclic hydrocarbons?
A) coal
B) liquid propane
C) natural gas
D) none of these
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The hydrocarbon model shown below could represent a saturated compound with the general formula CnH2n+2. 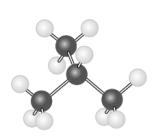
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following model of ethanol, CH3CH2OH. 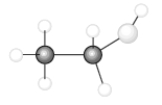 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-The number of carbon to hydrogen single bonds that are broken is_______.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-The number of carbon to hydrogen single bonds that are broken is_______.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not true about benzene?
A) has the formula C6H12
B) is a carcinogen
C) increases the octane rating of gasoline
D) is a cyclic hydrocarbon
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following model of ethanol, CH3CH2OH.  During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-Using the data in the following table,
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-Using the data in the following table, 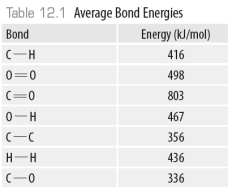 The amount of energy released in the formation of all the O-H bonds in the product water is ______kJ.
The amount of energy released in the formation of all the O-H bonds in the product water is ______kJ.
Correct Answer

verified
2802
2802 kJ
Correct Answer
verified
Multiple Choice
What term is used to describe the amount of heat energy released from burning?
A) heat of fusion
B) heat of vaporization
C) heat of combustion
D) heat of formation
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which process is used to increase the amount of the gasoline fraction in refining to match commercial demand?
A) reforming
B) oxygenating
C) cracking
D) gasification
F) C) and D)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Which of the following is not true about the compound MTBE?
A) it is an ether
B) made by fermentation
C) it is a carcinogen
D) used to oxygenate gasoline
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which hydrocarbon would be isolated at the top of a fractionation column?
A) C5H12
B) C10H22
C) C3H8
D) C18H36
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following model of ethanol, CH3CH2OH.  During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-In the balanced equation for the combustion of one mole of ethanol, the coefficient of water is _____.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-In the balanced equation for the combustion of one mole of ethanol, the coefficient of water is _____.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fractional distillation of petroleum
A) separates and purifies all of the hydrocarbons in crude oil.
B) separates fractions, each of which contain many hydrocarbons.
C) is the final step in refining gasoline.
D) fractions large molecules into smaller ones.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Butane [CH3CH2CH2CH3] and 2-methylpropane [(CH3) 2CHCH3] are
A) stereoisomers.
B) cis-trans isomers.
C) structural isomers.
D) optical isomers.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The fuel identified as M85 is
A) a substance with a molar mass of 85.
B) a fuel mixture of 85% methanol and 15% gasoline.
C) a fuel with a boiling point above 85 C.
D) a fuel that is 85% gasoline and 15% ethanol.
F) A) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which fossil fuel furnishes the most heat energy per gram?
A) petroleum
B) charcoal
C) natural gas
D) coal
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A coal gasification process involving a catalyst, carbon monoxide, hydrogen, steam and coal produces
A) only CO.
B) liquid petroleum.
C) octane.
D) methane and carbon dioxide.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 57
Related Exams