Correct Answer

verified
The sketch should show all of the approp...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Give the approximate bond angle for a molecule with a linear shape.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 60°
G) D) and E)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Place the following in order of decreasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. N2O NCl3 NO2⁻
A) NCl3 > NO2⁻ > N2O
B) NO2⁻ > N2O > NCl3
C) N2O > NO2⁻ > NCl3
D) NCl3 > N2O > NO2⁻
E) N2O > NCl3 > NO2⁻
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules have sp2 hybridization on the central atom? HCN SO2 OCl2 XeCl2
A) 4
B) 3
C) 2
D) 1
E) 0
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The VSEPR model predicts the H-N-H bond angle in NH2- to be
A) 60°.
B) 90°.
C) less than 109.5° but greater than 90°.
D) 109.5°.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of CF3+1.
A) eg = tetrahedral, mg = tetrahedral
B) eg = trigonal pyramidal, mg = trigonal pyramidal
C) eg = bent, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the hybridization for the Br in BrF5.
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with a tetrahedral shape.
A) 109.5°
B) 200°
C) 120°
D) 107°
E) 45°
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model,the molecular geometry of the central atom in ClO3- is
A) linear.
B) trigonal planar.
C) tetrahedral.
D) bent.
E) trigonal pyramidal.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of BrF4 -?
A) seesaw
B) square planar
C) square pyramidal
D) pyramidal
E) trigonal bipyramidal
G) A) and E)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
How many of the following molecules have sp3d hybridization on the central atom? CCl4 BrF5 AsF5 BrF3
A) 2
B) 0
C) 4
D) 1
E) 3
G) A) and E)
Correct Answer

verified
A
Correct Answer
verified
Essay
Use molecular orbital theory to determine whether He22⁺ or He2⁺ is more stable.Draw the molecular orbital diagram for each and explain your answer.
Correct Answer

verified
The MO diagram should show He22⁺ with 2 el...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Using the VSEPR model,the molecular geometry of the central atom in NH4+ is
A) linear.
B) trigonal planar.
C) tetrahedral.
D) bent.
E) trigonal pyramidal.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Matching
Match the following.
Correct Answer
Multiple Choice
Choose the compound below that contains at least one polar covalent bond,but is nonpolar.
A) GeH2Br2
B) SBr2
C) AsBr5
D) CBr2Cl2
E) All of the above are nonpolar and contain a polar covalent bond.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the number of electron groups around a molecule with sp hybridization.
A) 1
B) 2
C) 3
D) 4
E) 5
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an oil tanker leaks oil in the ocean,where does the oil go?
A) The oil floats on the seawater; water is polar and oil is nonpolar.
B) The oil mixes with the water.
C) The oil sinks below the seawater; water is nonpolar and oil is polar.
D) The oil floats on the seawater; water is nonpolar and oil is polar.
E) The oil sinks below the seawater; water is polar and oil is nonpolar.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is most stable. 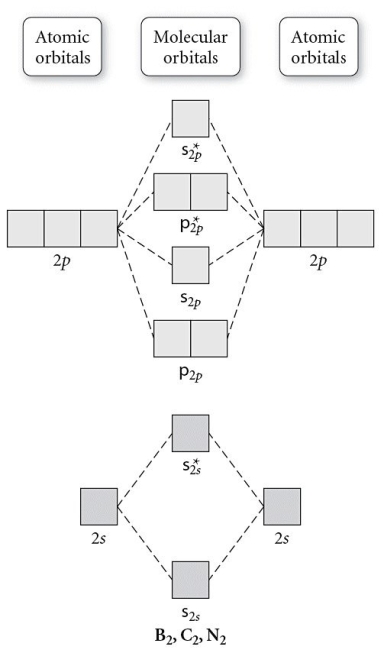
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Determine the molecular geometry about each interior atom in the following structure.Sketch the three-dimensional structure and label the interior atoms with the corresponding molecular geometry. CH2CHCCCH3
Correct Answer

verified
The sketch should show all of the approp...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of BH3.
A) eg = trigonal planar, mg = trigonal planar
B) eg = tetrahedral, mg = trigonal planar
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal planar, mg = tetrahedral
E) eg = trigonal bipyramidal, mg = trigonal bipyramidal
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 144
Related Exams