A) London dispersion forces
B) hydrogen bonding
C) ion-dipole forces
D) dipole-dipole forces
E) ionic bonding
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Molecules with ________ do not generally exhibit liquid-crystalline properties because they lack the rigidity necessary for alignment.
A) both single and double bonds
B) double or triple bonds
C) single, double, and triple bonds
D) only single bonds
E) only single and double bonds
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following should have the lowest boiling point?
A) CH4
B) PCl3
C) C2H5COOH
D) LiCl
E) Cl2S
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statements about viscosity are true? (i) Viscosity increases as temperature decreases. (ii) Viscosity increases as molecular weight increases. (iii) Viscosity increases as intermolecular forces increase.
A) (i) only
B) (ii) and (iii)
C) (i) and (iii)
D) none
E) all
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The phase diagram of a substance is shown above.The area labeled ________ indicates the liquid phase for the substance.
A) w
B) x
C) y
D) z
E) y and z
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the phase diagram shown above,what is the normal boiling point (°C) ?
A) 10
B) 20
C) 30
D) 40
E) 50
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The intermolecular force(s) responsible for the fact that CH4 has the lowest boiling point in the set CH4,CH3CH3,CH3CH2CH3,CH3CH2CH2CH3 is/are ________.
A) hydrogen bonding
B) London dispersion forces
C) mainly hydrogen bonding but also dipole-dipole interactions
D) dipole-dipole interactions
E) mainly London-dispersion forces but also dipole-dipole interactions
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has hydrogen bonding as its only intermolecular force?
A) HCl
B) NH3
C) H2O
D) CH3OH
E) None, all of the above exhibit dispersion forces.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What types of intermolecular forces exist between CH3OH and H2O?
A) dipole-dipole and ion-dipole
B) dispersion forces, dipole-dipole, and hydrogen bonding
C) dispersion forces, dipole-dipole, and ion-dipole
D) dispersion forces, hydrogen bonding, dipole-dipole, and ion-dipole
E) dispersion forces and ion-dipole
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Octane C8H18 molecules are held together by ________.
A) ion-ion interactions
B) hydrogen bonding
C) ion-dipole interactions
D) London dispersion forces
E) dipole-dipole interactions
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the common types of smectic liquid-crystalline phases?
A) A, C, and D
B) A only
C) A and C
D) A and D
E) C and D
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following are characteristics of liquid crystal behavior except ________.
A) long axial structure
B) carbon-carbon single bonds
C) double bonding
D) ionic configuration
E) polar groups
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type(s) of intermolecular forces exist between Cl2 and CCl4?
A) dispersion forces and ion-dipole
B) dispersion forces and dipole-dipole
C) dispersion forces
D) dispersion forces, ion-dipole, and dipole-dipole
E) None. Since both are gases at room temperature, they do not interact with each other.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Together,liquids and solids constitute ________ phases of matter.
A) the compressible
B) the fluid
C) the condensed
D) all of the
E) the disordered
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A volatile liquid is one that ________.
A) is highly flammable
B) is highly viscous
C) is highly hydrogen-bonded
D) is highly cohesive
E) readily evaporates
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heating curve shown was generated by measuring the heat flow and temperature of a solid as it was heated.The heat flow into the sample in the segment ________ will yield the value of the ΔHfusion of this substance.
A) AB
B) BC
C) CD
D) DE
E) EF
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the figure above,the boiling point of diethyl ether under an external pressure of 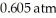 is ________ °C.
is ________ °C.
A) 40
B) 10
C) 30
D) 20
E) 0
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the phase diagram shown above,what is the normal melting point (°C) ?
A) -13
B) 0
C) 38
D) 10
E) 29
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following substances,________ has the highest boiling point.
A) HOCH2CH2CH2OH
B) CH3CH2OH
C) C4H10
D) N2
E) Cl2
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule has the lowest boiling point?
A) CH3CH2CH2CH3
B) CH3CH2CH3
C) CH3CH3
D) CH4
E) H2O
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 124
Related Exams