A) Si
B) S
C) Ar
D) Ca
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A bottle of pure element was missing part of a label.The label said 2.258 x 1023 atoms.You determine the mass of the elements in the bottle to be 10.51946.What is the identity of this element?
A) B
B) N
C) Si
D) Sr
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element indicated by letter in the following periodic table is a gas at room temperature and a pressure of 1.0 atm? 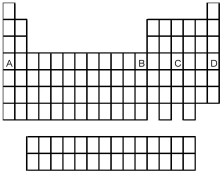
A) A
B) B
C) C
D) D
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element can form more than one kind of monatomic ion?
A) Sr
B) Al
C) Sn
D) O
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds contains ionic bonds?
A) MgS
B) HF
C) NCl3
D) SiO2
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The symbol that is usually used to represent atomic number is
A) A.
B) N.
C) X.
D) Z.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is most likely to form a binary oxide with the formula MO (where M = element A,B,C,orD) ?
A) element A
B) element B
C) element C
D) element D
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the periodic table below to answer the following questions. 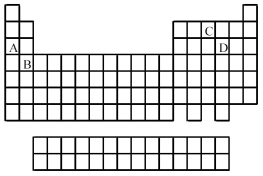 -Which elements commonly form anions?
-Which elements commonly form anions?
A) A and B
B) A and C
C) B and D
D) C and D
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope represented by 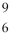 C is named
C is named
A) carbon-6.
B) carbon-3.
C) carbon-9.
D) carbon-15.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element indicated by letter in the following periodic table is the poorest conductor of electricity and heat? 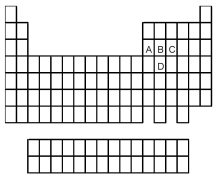
A) A
B) B
C) C
D) D
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following drawings depicts a chemical reaction consistent with Dalton's atomic theory? 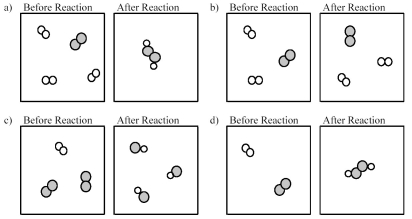
A) drawing a)
B) drawing b)
C) drawing c)
D) drawing d)
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The definitive distinction between ionic bonding and covalent bonding is that
A) ionic bonding involves a sharing of electrons and covalent bonding involves a transfer of electrons.
B) ionic bonding involves a transfer of electrons and covalent bonding involves a sharing of electrons.
C) ionic bonding requires two nonmetals and covalent bonding requires a metal and a nonmetal.
D) covalent bonding requires two nonmetals and ionic bonding requires a metal and a nonmetal.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the chemical symbol for carbon?
A) Co
B) Cr
C) C
D) Ca
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following drawings depicts a chemical reaction consistent with Dalton's atomic theory? 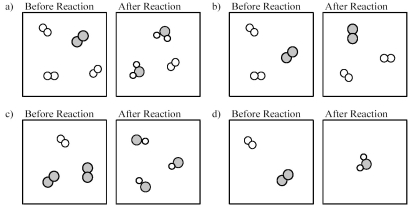
A) drawing a)
B) drawing b)
C) drawing c)
D) drawing d)
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element indicated by letter in the following periodic table reacts rapidly with water to form an alkaline solution? 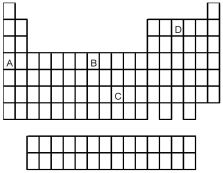
A) A
B) B
C) C
D) D
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The charge-to-mass ratio of an electron was established by
A) Millikan's oil drop experiment.
B) Rutherford's gold foil experiment.
C) Thomson's cathode ray tube experiment.
D) None of these
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What group of elements does the shaded area in the following periodic table indicate? 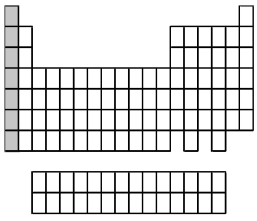
A) alkali metals
B) alkaline earth metals
C) halogens
D) noble gases
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following figures represents 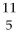 B? Unshaded spheres represent neutrons and shaded spheres represent protons.
B? Unshaded spheres represent neutrons and shaded spheres represent protons. 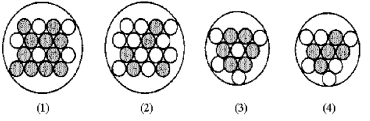
A) figure (1)
B) figure (2)
C) figure (3)
D) figure (4)
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following elements are nonmetals except
A) beryllium.
B) carbon.
C) hydrogen.
D) oxygen.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which are isotopes? An atom that has an atomic number of 12 and a mass number of 26 is an isotope of an atom that has
A) an atomic number of 13 and a mass number of 26.
B) an atomic number of 12 and a mass number of 24.
C) 12 neutrons and 14 protons.
D) 12 protons and 14 neutrons.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 161 - 180 of 275
Related Exams