A) 1.66
B) 2.77
C) 11.23
D) 11.14
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider a solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride. The concentration of hydrogen fluoride after addition of 9.00 mL of 0.0100 M HCl to 25.0 mL of this solution is ________ M.
A) 0.0953
B) 0.0900
C) 0.130
D) 0.122
E) 0.00976
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following table of 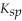 values.
values. 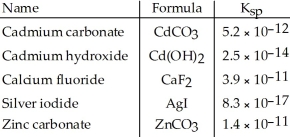 -Which compound listed below has the smallest molar solubility in water?
-Which compound listed below has the smallest molar solubility in water?
A) ZnCO3
B) Cd(OH) 2
C) CdCO3
D) AgI
E) CaF2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution containing which one of the following pairs of substances will be a buffer solution?
A) KI, HI
B) AgBr, HBr
C) CuCl, HCl
D) CsI, HI
E) none of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 25.0 mL sample of an HCl solution is titrated with a 0.139 M NaOH solution. The equivalence point is reached with 15.4 mL of base. The concentration of HCl is ________ M.
A) 11.7
B) 0.00214
C) 0.0856
D) 0.267
E) 0.139
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the percent ionization of nitrous acid in a solution that is 0.260 M in nitrous acid. The acid dissociation constant of nitrous acid is 4.50 × 10-4.
A) 1.17 × 10-4
B) 0.0450
C) 4.16
D) 0.314
E) 5.78
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The concentration of iodide ions in a saturated solution of silver iodide is ________ M. The solubility product constant of AgI is 8.3 × 10-17.
A) 3.8 × 10-11
B) 3.0 × 10-10
C) 9.1 × 10-9
D) 3.5 × 10-9
E) 1.4 × 10-8
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider a solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride. The concentration of fluoride ions after the addition of 9.00 mL of 0.0100 M HCl to 25.0 mL of this solution is ________ M.
A) 0.0735
B) 0.0762
C) 0.0980
D) 0.0709
E) 0.00253
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the maximum concentration (in M) of silver ions (Ag+) in a solution that contains 0.025 M of CO32-. The Ksp of Ag2CO3 is 8.1 × 10-12.
A) 1.8 × 10-5
B) 1.4 × 10-6
C) 2.8 × 10-6
D) 3.2 × 10-10
E) 8.1 × 10-12
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the percent ionization of formic acid (HCO2H) in a solution that is 0.322 M in formic acid and 0.178 M in sodium formate (NaHCO2) . The Ka of formic acid is 1.77 × 10-4.
A) 35.6
B) 0.1011
C) 10.8
D) 1.03 × 10-3
E) 3.488
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solubility of manganese (II) hydroxide (Mn(OH) 2) is 2.2 × 10-5 M. What is the Ksp of Mn(OH) 2?
A) 1.1 × 10-14
B) 4.3 × 10-14
C) 2.1 × 10-14
D) 4.8 × 10-10
E) 2.2 × 10-5
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the maximum concentration (in M) of magnesium ions (Mg+2) in a solution that contains 0.025 M of CO32-. The Ksp of MgCO3 is 3.5 × 10-8.
A) 1.8 × 10-5
B) 1.4 × 10-6
C) 2.8 × 10-6
D) 3.2 × 10-10
E) 8.1 × 10-12
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a solution prepared by dissolving 0.250 mol of benzoic acid (C7H5O2H) and 0.150 mol of sodium benzoate (NaC7H5O2) in water sufficient to yield 1.00 L of solution. The Ka of benzoic acid is 6.50 × 10-5.
A) 4.409
B) 3.965
C) 10.035
D) 9.591
E) 5.190
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following could be added to a solution of acetic acid to prepare a buffer?
A) sodium acetate only
B) sodium acetate or sodium hydroxide
C) nitric acid only
D) hydrofluoric acid or nitric acid
E) sodium hydroxide only
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following pairs cannot be mixed together to form a buffer solution?
A) HONH2, HONH3Cl
B) NaCl, HCl
C) RbOH, HF
D) KOH, HNO2
E) H2SO3, KHSO3
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following aqueous solutions would you expect AgI to have the highest solubility?
A) pure water
B) 0.050 M BaI2
C) 0.050 M NaI
D) 0.050 M KI
E) 0.010 M AgNO3
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The concentration of fluoride ions in a saturated solution of barium fluoride is ________ M. The solubility product constant of BaF2 is 1.7 × 10-6.
A) 3.8 × 10-4
B) 3.0 × 10-3
C) 1.5 × 10-2
D) 7.5 × 10-3
E) 1.4 × 10-4
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the solubility (in M) of PbCl2 in a 0.15 M solution of HCl? The Ksp of PbCl2 is 1.6 × 10-5.
A) 2.0 × 10-3
B) 1.1 × 10-4
C) 1.8 × 10-4
D) 7.1 × 10-4
E) 1.6 × 10-5
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following aqueous solutions would you expect AgBr to have the lowest solubility?
A) 0.040 M SrBr2
B) pure water
C) 0.040 M NaBr
D) 0.040 M KBr
E) 0.010 M AgNO3
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the principal organs that regulate the pH of the carbonic acid-bicarbonate buffer system in the blood?
A) kidneys, liver
B) lungs, kidneys
C) spleen, liver
D) lungs, skin
E) brain stem, heart
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 116
Related Exams