A) AB
B) BC
C) CD
D) DE
E) EF
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
In general, intramolecular forces determine the ________ properties of a substance and intermolecular forces determine its ________ properties.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the ________ liquid crystalline phase, the component molecules are aligned along their long axis and are arranged in layers with the molecules in each plane twisted slightly in relation to the molecules in the planes above and below.
A) nematic
B) smectic A
C) smectic B
D) smectic C
E) cholesteric
G) B) and E)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Which one of the following should have the lowest boiling point?
A) CF4
B) SnF3
C) C4H9OH
D) HCl
E) F2O
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following substances will have hydrogen bonding as one of its intermolecular forces?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
With what compound will NH3 experience only ion-dipole intermolecular forces?
A) LiCl
B) SiH4
C) CH3I
D) C3H7OH
E) OCl2
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
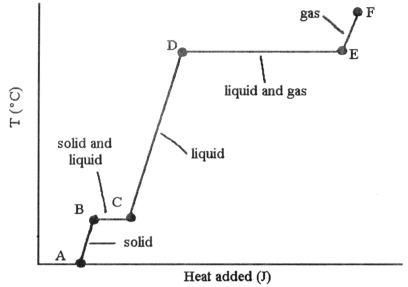 -The heating curve shown was generated by measuring the heat flow and temperature of a solid as it was heated. The heat flow into the sample in the segment ________ will yield the value of the ΔHfusion of this substance.
-The heating curve shown was generated by measuring the heat flow and temperature of a solid as it was heated. The heat flow into the sample in the segment ________ will yield the value of the ΔHfusion of this substance.
A) AB
B) BC
C) CD
D) DE
E) EF
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The principal source of the difference in the normal boiling points of ICl (97 °C; molecular mass 162 amu)and Br2 (59 °C; molecular mass 160 amu)is both dipole-dipole interactions and London dispersion forces.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
C12H26 molecules are held together by ________.
A) ion-ion interactions
B) hydrogen bonding
C) ion-dipole interactions
D) dipole-dipole interactions
E) dispersion forces
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
 -The heating curve shown was generated by measuring the heat flow and temperature for a solid as it was heated. The slope of the ________ segment corresponds to the heat capacity of the solid.
-The heating curve shown was generated by measuring the heat flow and temperature for a solid as it was heated. The slope of the ________ segment corresponds to the heat capacity of the solid.
A) AB
B) BC
C) CD
D) DE
E) EF
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The predominant intermolecular force in CaBr2 is ________.
A) London-dispersion forces
B) ion-dipole forces
C) ionic bonding
D) dipole-dipole forces
E) hydrogen bonding
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has hydrogen bonding as its only intermolecular force?
A) NH3
B) H2O
C) C3H7OH
D) HOCH2CH2OH
E) None, all of the above exhibit dispersion forces.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the following information, which compound has the strongest intermolecular forces? Substance ΔHvap (kJ/mol) Argon (Ar) 6.3 Benzene (C6H6) 31.0 Ethanol (C2H5OH) 39.3 Water (H2O) 40.8 Methane (CH4) 9.2
A) Argon
B) Benzene
C) Ethanol
D) Water
E) Methane
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A volatile liquid is one that ________.
A) is highly flammable
B) is highly viscous
C) is highly hydrogen-bonded
D) is highly cohesive
E) readily evaporates
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which type of liquid crystal is colored and changes color with temperature?
A) nematic
B) smectic A
C) cholesteric
D) smectic B
E) smectic C
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Elemental iodine (I2) is a solid at room temperature. What is the major attractive force that exists among different I2 molecules in the solid?
A) London dispersion forces
B) dipole-dipole rejections
C) ionic-dipole interactions
D) covalent-ionic interactions
E) dipole-dipole attractions
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When NaCl dissolves in water, aqueous Na+ and Cl- ions result. The force of attraction that exists between Na+ and H2O is called a(n) ________ interaction.
A) dipole-dipole
B) ion-ion
C) hydrogen bonding
D) ion-dipole
E) London dispersion force
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
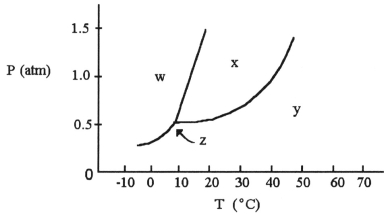 -The normal melting point of the substance with the phase diagram shown above is ________ °C.
-The normal melting point of the substance with the phase diagram shown above is ________ °C.
A) 15
B) 25
C) 35
D) 45
E) 55
G) A) and C)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What is the predominant intermolecular force in CH4?
A) London-dispersion forces
B) ion-dipole attraction
C) ionic bonding
D) dipole-dipole attraction
E) hydrogen bonding
G) None of the above
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What types of intermolecular forces exist between HI and H2S?
A) dipole-dipole and ion-dipole
B) dispersion forces, dipole-dipole, and ion-dipole
C) dispersion forces, hydrogen bonding, dipole-dipole, and ion-dipole
D) dispersion forces and dipole-dipole
E) dispersion forces and ion-dipole
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 124
Related Exams