A) [Co(H2O) 6]2+
B) [Co(NH3) 6]2+
C) [CoF6]4-
D) [Co(CN) 6]4-
E) [Co(en) 6]2+
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct formula for sodium tetracyanonickelate(II) ion is:
A) Ni(CN) 4
B) [Ni(CN) 4]+
C) [Ni(CN) 4]2+
D) [Ni(CN) 4]-
E) [Ni(CN) 4]2-
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The chemical formula of the dibromobis(oxalato)cobaltate(III) ion is: [CoBr2(C2O4)2]3-
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of Co in the complex [Co(H2O) 4Cl2]Cl
A) +1
B) +2
C) +3
D) +4
E) None of the above
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Copper can be separated from iron in the ore chalcopyrite (CuFeS2) by heating the ore in the presence of oxygen to form copper(I) sulfide, iron(II) oxide, and one other product. Complete the following partially balanced equation by deciding both the identity of the missing compound and the number of moles required to balance the equation. (Note, no other coefficients are allowed to be changed.) 2CuFeS2(s) + 4O2(g) Cu2S(s) + 2FeO(s) + _____
A) 2SO3(g)
B) 3SO3(g)
C) 2SO2(g)
D) 3SO2(g)
E) None of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the coordination compound [Cr(NH3) (en) 2Cl]Br2, the coordination number (C.N.) and oxidation number (O.N.) of the metal atom are, respectively,
A) C.N. = 6; O.N. = +4.
B) C.N. = 6; O.N. = +3.
C) C.N. = 5; O.N. = +2.
D) C.N. = 4; O.N. = +2.
E) C.N. = 4; O.N. = +3.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine
(i) the oxidation number of the metal,
(ii) the number of d electrons,
(iii) the coordination number,
(iv) the charge of the complex ion, and
(v) the number and type of ligands for the coordination compound shown below. 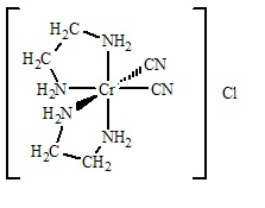
A) i = +2; ii = 3 d electrons; iii = 5; iv = 1+; v = zero monodentate and four bidentate
B) i = +3; ii = 2 d electrons; iii = 6; iv = 1+; v = two monodentate and two bidentate
C) i = +2; ii = 3 d electrons; iii = 5; iv = 1+; v = two monodentate and one bidentate
D) i = +3; ii = 3 d electrons; iii = 6; iv = 1+; v = two monodentate and two bidentate
E) None of the above have all five answers correctly presented
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these square planar complex ions can have cis-trans isomers
A) [Pt(NH3) 4]2+
B) [Ni(NH3) 4]2+
C) [Pt(NH3) 2Cl2]
D) [Pt(NH3) Cl3]-
E) [Ni(NH3) 3Cl]+
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which response gives the correct coordination number (C.N.) and oxidation number (O.N.) of the transition metal atom in [Co(NH3) 2(H2O) 2Cl2]+
A) C.N. = 2; O.N. = +3
B) C.N. = 3; O.N. = +1
C) C.N. = 4; O.N. = +2
D) C.N. = 6; O.N. = +1
E) C.N. = 6; O.N. = +3
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The correct name of the complex ion [Co(H2O)4Cl2]+ is: tetraaquodichlorocobalt(III) ion
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these ligands produces the strongest crystal field
A) Cl-
B) CO
C) OH-
D) H2O
E) NH3
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the coordination compound [Pt(NH3) 2Cl2], the coordination number and oxidation number of the central atom are, respectively,
A) 2, 0.
B) 4, 4.
C) 5, 0.
D) 4, 2.
E) 6, 2.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the number of unpaired electrons in the [Fe(CN) 6]3- ion.
A) 0
B) 1
C) 3
D) 5
E) None of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the coordination number of chromium in Cr(en) 2Cl2
A) 2
B) 4
C) 6
D) 8
E) None of the above
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
A molecule or atom that accepts an electron pair to form a coordinate covalent bond is called a Lewis base.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron configuration of a Cr3+ ion is
A) [Ar]3d5.
B) [Ar]4s13d2.
C) [Ar]3d3.
D) [Ar]4s13d5.
E) [Ar]4s23d4.
G) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
The oxidation number of Co in [Co(NH3)4Cl2]Cl is +1.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the complex ion [Fe(CN) 6]4-, the oxidation number of Fe is
A) +1.
B) +2.
C) +3.
D) -4.
E) +6.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In K4[Fe(CN) 6], how many 3d electrons does the iron atom have
A) 3
B) 4
C) 5
D) 6
E) 7
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In Na3[Ni(SCN) 5], how many 3d electrons does nickel have
A) 6
B) 7
C) 8
D) 9
E) 10
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 92
Related Exams