A) H2
B) O2
C) Ne
D) N2
E) CO
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 0.3781 g of a gas with a molecular weight of 18.015 g mol-1 is in a ridged 5.00 L container at 384 K, calculate the final pressure. Assume ideal behaviour.
A) 0.682 bar
B) 0.109 bar
C) 0.0867 bar
D) 0.134 bar
E) 0.0963 bar
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of CO are contained in a 5.00 L tank at 155 °C and 2.80 bar?
A) 0.393 moles
B) 1.10 moles
C) 2.51 moles
D) 0.455 moles
E) 0.289 moles
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Define effusion.
Correct Answer

verified
Effusion is the proc...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The atmospheric pressure is 715 mmHg. What is the pressure in bar?
A) 1.10 bar
B) 0.940 bar
C) 1.08 bar
D) 0.9533 bar
E) 0.9197 bar
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the average speed (actually the root mean square speed) of a neon atom at 27 °C?
A) 5.78 m s-1
B) 19.3 m s-1
C) 183 m s-1
D) 609 m s-1
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mole fraction of nitrous oxide in dry air near sea level is 1.818 × 10-5, where the molar mass of nitrous oxide is 44.013. The concentration of nitrous oxide in the atmosphere is ________ ppm.
A) 2.0 × 1012
B) 5.0 × 10-5
C) 500
D) 18.18
E) 5.0 × 10-13
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the gases in the graph below has the largest molar mass? 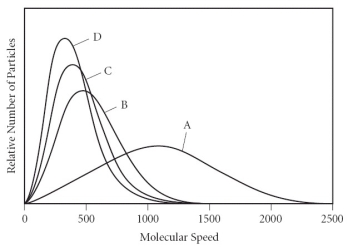
A) A
B) B
C) C
D) D
E) There is not enough information to determine the answer.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the density of CO2 gas at STP.
A) 1.94 g L-1
B) 1.80 g L-1
C) 2.24 g L-1
D) 4.46 g L-1
E) 5.10 g L-1
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following samples has the greatest density at STP?
A) NO2
B) Xe
C) SO2
D) SF6
E) All of these samples have the same density at STP.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a gas is proportional to number of moles of a gas. What is this known as?
A) Avogadro's law
B) Ideal gas law
C) Charles's law
D) Boyle's law
E) Dalton's law
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
This equation is used to calculate the properties of a gas under nonideal conditions.
A) Charles's law
B) Avogadro's law
C) Boyle's law
D) van der Waals equation
E) Dalton's law
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -Dalton's law
A) measures blood pressure
B) measures pressure of a gas
C) V1/n1 = V2/n2
D) PV = nRT
E) P1V1 = P2V2
F) measures atmospheric pressure
G) V1/T1 = V2/T2
H) PT = PA + PB + PC ...
J) A) and G)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ratio of the rate of effusion of oxygen to an unknown gas is 0.935. What is the other gas?
A) Ne
B) Ar
C) F2
D) N2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the volume of 9.783 × 1023 atoms of He at 9.25 bar and 512 K?
A) 7.48 L
B) 3.69 L
C) 1.85 L
D) 15.4 L
E) 30.8 L
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the root mean square velocity of nitrogen molecules at 25 °C.
A) 729 m s-1
B) 515 m s-1
C) 149 m s-1
D) 297 m s-1
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The action of some commercial drain cleaners is based on the following reaction: 2NaOH(s) + 2Al(s) + 6H2O(l) → 2NaAl(OH) 4(s) + 3H2(g) What is the volume of H2 gas formed at STP when 6.32 g of Al reacts with excess NaOH?
A) 3.50 L
B) 5.25L
C) 7.98 L
D) 8.59L
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gases has the highest root mean square velocity at constant temperature? NH3 CO2 NO2 F2 O3
A) NH3
B) CO2
C) NO2
D) F2
E) O3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At what temperature does argon have a root mean square velocity of 492 m s-1?
A) 321 K
B) 291 K
C) 340 K
D) 388 K
E) 409 K
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gases has the lowest average speed at 25 °C?
A) C3H8
B) Kr
C) CH3NH2
D) SO2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 154
Related Exams