Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ion with an atomic number of 34 and 36 electrons has a ________ charge.
A) -2
B) +34
C) -36
D) +2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules is nonpolar?
A) NH3
B) CH3Cl
C) CCl4
D) H2O
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A bond where the electrons are shared unequally is called a(n) :
A) polar covalent
B) coordinate covalent
C) purely (nonpolar) covalent
D) ionic
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Complete the following table.
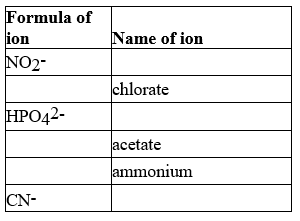
Correct Answer

verified
line 1)nitrite
line ...View Answer
Show Answer
Correct Answer
verified
line ...
View Answer
Multiple Choice
The reaction of calcium and sulfur produces:
A) Ca2S
B) CaS2
C) CaS
D) Ca2S3
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which quantity contains the fewest moles?
A) 10 g N2
B) 10 g CO
C) 10 g Si
D) 10 g AlH3
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following anions has the same charge as the hydroxide ion?
A) NO3-
B) SO4-2
C) CO3-2
D) PO4-3
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these molecules has trigonal pyramidal molecular geometry?
A) NF3
B) BF3
C) SO3
D) AlF3
F) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
The molar mass of an element in grams is numerically equal to that element's atomic mass in amu.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the electronegativities below, which of the following covalent single bonds is the most polar? Element: H C N O Electronegativity 2.1 2.5 3.0 3.5
A) C-H
B) N-C
C) O-N
D) O-C
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many protons and electrons are there in the calcium ion?
A) 20 protons, 20 electrons
B) 20 protons, 22 electrons
C) 20 protons, 18 electrons
D) 20 protons, 19 electrons
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules is polar?
A) CH4
B) CO2
C) SO2
D) SO3
F) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
Hydrogen sulfide (H2S)is a covalent compound.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
An atom ________ electrons when it forms a cation.
Correct Answer

verified
Correct Answer
verified
Short Answer
Draw the Lewis structures for the following compounds. Draw all possible resonance structures. What is the molecular geometry and the polarity of each compound? A)CO32- B)NF3 C)CHCl3 D)SO2
Correct Answer

verified
Correct Answer
verified
Short Answer
A covalent bond involves ________ electrons between atoms.
Correct Answer

verified
Correct Answer
verified
True/False
2 moles of CH3CH2CH2CH3 contains 5 moles of H2 molecules.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Ammonium nitrate is an ionic compound.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
The outer electron shell is called the ________ shell.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 107
Related Exams