A) 18 g
B) 1.9 g
C) 6.6 g
D) 12 g
E) 1.2 g
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the atoms of 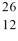 Mg and
Mg and 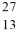 Al. Both of these species have the same
Al. Both of these species have the same
A) number of neutrons and electrons.
B) number of ions.
C) number of neutrons.
D) number of neutrons and mass number.
E) number of protons and electrons.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following postulates from Dalton's atomic theory is incorrectly stated?
A) The atoms in a given sample of an element are identical.
B) Matter consists of tiny particles called atoms.
C) In chemical reactions, atoms merely rearrange and do not disintegrate.
D) In a given chemical compound, the atoms can be present in various numerical ratios.
E) The atoms of different elements differ in mass and other properties.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
A compound of phosphorus and chlorine contains 3.00 grams of phosphorus and 10.3 grams of chlorine. There are ________ grams of phosphorus in a sample of the compound containing 17.2 grams of chlorine. Hint: Consider the ratio of the masses to determine the mass of phosphorous.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The species 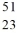 V has the same number of neutrons as
V has the same number of neutrons as
A) ![]() V
V
B) ![]() Sc
Sc
C) ![]() Mn
Mn
D) ![]() Cr
Cr
E) ![]() Co
Co
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Uranium exists in nature in the form of several isotopes and the different isotopes have different
A) atomic numbers.
B) charges.
C) numbers of electrons.
D) numbers of neutrons.
E) numbers of protons.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
If 2.00 grams of molecular hydrogen react with 16.00 grams of oxygen to form water, how many grams of water must be formed if all of the hydrogen and oxygen react? Hint: Consider the ratio of the masses to determine the mass of compound.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following have roughly the same mass?
A) a proton and an electron
B) a neutron and an electron
C) a neutron and a proton
D) an electron and an alpha particle
E) none of these options
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
To form hydrogen sulfide, H2S, 4.03 g of molecular hydrogen (H2) are reacted with 62.13 g of sulfur (S) . If all of the hydrogen and sulfur completely react to form hydrogen sulfide, how many grams of hydrogen sulfide should be formed?
A) 66.16 g
B) 58.10 g
C) 4.03 g
D) 70.19 g
E) 33.03 g
G) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Molecules are made of tiny particles called ________.
Correct Answer

verified
Correct Answer
verified
True/False
When an electrical spark is passed through hydrogen gas molecules, positive ions can be generated through the interaction.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The species shown below that has 24 electrons is
A) ![]() Cr
Cr
B) ![]() Mn
Mn
C) ![]() Mg
Mg
D) ![]() Sc
Sc
E) ![]() V
V
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic mass of naturally occurring gallium, which is a mixture of two isotopes, is listed as 69.723 u. This means that ,Hint: Individual atoms have a mass number that is the sum of the protons and neutrons.
A) all gallium atoms found in nature have a mass that is 69.723/12.000 times as great as that of a 12C atom.
B) all gallium atoms found in nature have a mass that is 69.723/1.0079 times as great as that of a 1H atom.
C) some gallium atoms found in nature have a mass that is 69.723/12.000 times as great as that of a 12C atom.
D) some gallium atoms found in nature have a mass that is 69.723/1.0079 times as great as that of a 1H atom.
E) no gallium atoms found in nature has a mass that is 69.723/12.000 times as great as that of a 12C atom.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
A compound is made of nitrogen and hydrogen in a ratio of 5.65 grams nitrogen to 1.22 grams of hydrogen. There are ________ grams of nitrogen in a sample of this compound containing 4.00 grams of hydrogen. Hint: Consider the ratio of the masses to determine the mass of nitrogen.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following examples is consistent with the postulates from Dalton's atomic theory? Hint: Be sure to keep the definitions of atoms and molecules straight!
A) The atoms in a sample of chlorine are similar to the atoms in a sample of elemental sulfur.
B) Matter consists of extremely tiny particles that are always either positively or negatively charged.
C) When water is formed from oxygen and hydrogen molecules, the atoms in water are grouped differently compared to the atoms in molecules of hydrogen and oxygen.
D) A sample of water contains hydrogen and oxygen atoms combined in two different ratios by mass.
E) There are eight different types of sulfur atoms in any naturally occurring sample of elemental sulfur.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following particles will not be deflected by charged plates?
A) hydrogen atoms
B) cathode rays
C) alpha particles
D) protons
E) These are all deflected by charged plates.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Atoms must be broken apart into their subatomic particles and then rearranged in order for chemical reactions to occur. Hint: What happens in a chemical reaction between reactants and products?
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Compare 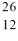 Mg and
Mg and 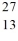 Al. In what respect do these species differ? I. number of neutrons and number of electrons
II. number of protons, and number of neutrons
III. mass number and number of protons
Al. In what respect do these species differ? I. number of neutrons and number of electrons
II. number of protons, and number of neutrons
III. mass number and number of protons
A) I only
B) II only
C) III only
D) I and III
E) I, II, and III
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
Average atomic masses are used in the periodic table. These values give the true representation of mixtures of isotopes for different elements that are consistent around the world and universe.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is/are consistent with Dalton's atomic theory? I. The atoms in a given sample of an element do not share any common properties. II. Matter consists of particles called atoms. III. In chemical reactions, atoms merely rearrange and do not disintegrate.
A) III only
B) II only
C) I only
D) II and III
E) I and III
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 90
Related Exams