A) have a branching carbon skeleton
B) have different combinations of double bonds between carbon atoms
C) have different positions of double bonds between carbon atoms
D) form enantiomers
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is carbon so important in biology?
A) It is a common element on Earth.
B) It has very little electronegativity, making it a good electron donor.
C) It bonds to only a few other elements.
D) It can form a variety of carbon skeletons and host functional groups.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules is polar? C₃H₇OHC₂H₅COOH
A) C₃H₇OH and C₂H₅COOH are both polar molecules.
B) Neither C₂H₅COOH or C₃H₇OH is polar.
C) C₂H₅COOH is polar, but C₃H₇OH is not polar.
D) C₂H₅COOH is not polar, but C₃H₇OH is polar.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that ________.
A) have identical chemical formulas but differ in the branching of their carbon skeletons
B) are mirror images of each other
C) differ in the location of their double bonds
D) differ in the arrangement of atoms around their double bonds
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figure to answer the question.
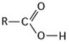 What is the name of the functional group shown in the figure?
What is the name of the functional group shown in the figure?
A) carbonyl
B) ketone
C) aldehyde
D) carboxyl
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
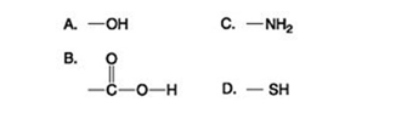 Which functional group shown can pick up protons and raise the pH of the surrounding solution?
Which functional group shown can pick up protons and raise the pH of the surrounding solution?
A) A
B) B
C) C
D) D
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the functional groups is not reactive but serves as a recognizable tag on the DNA molecule and alter the expression of genes in the cells.
A) amino
B) methyl
C) carboxyl
D) hydroxyl
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some carbon skeletons have different numbers and locations of double bonds to ________.
A) add molecular complexity and diversity that characterize living matter
B) be more flexible that makes the molecule stronger
C) stay in its liquid state
D) increase its solubility in water
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following illustrations is not a structural isomer of an organic compound with the molecular formula C₆H₁₄? For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element present in all organic molecules is ________.
A) hydrogen
B) oxygen
C) carbon
D) nitrogen
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following carbon molecules does not have the bond angle of 109.5°?
A) CH₄
B) C₂H₄
C) C₂H₆
D) C₃H₈
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the question.
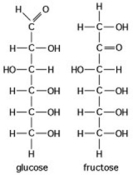 The figure shows the structures of glucose and fructose. These two molecules differ in the ________.
The figure shows the structures of glucose and fructose. These two molecules differ in the ________.
A) number of carbon, hydrogen, and oxygen atoms
B) types of carbon, hydrogen, and oxygen atoms
C) arrangement of carbon, hydrogen, and oxygen atoms
D) number of oxygen atoms joined to carbon atoms by double covalent bonds
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the question.
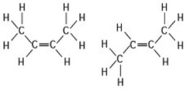 The two molecules shown in the figure are best described as ________.
The two molecules shown in the figure are best described as ________.
A) enantiomers
B) radioactive isotopes
C) structural isomers
D) cis-trans isomers
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true of carbon?
A) It forms only polar molecules.
B) It can form a maximum of three covalent bonds with other elements.
C) It is highly electronegative.
D) It can form both polar and nonpolar bonds.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Differences among organisms are caused by differences in the ________.
A) elemental composition from organism to organism
B) types and relative amounts of organic molecules synthesized by each organism
C) sizes of the organic molecules in each organism
D) types of inorganic compounds present in each organism
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons does one atom of carbon share to complete its valence shell?
A) 2
B) 3
C) 4
D) 8
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other? Testosterone and estradiol ________.
A) are structural isomers but have the same molecular formula
B) are cis-trans isomers but have the same molecular formula
C) have different functional groups attached to the same carbon skeleton
D) are enantiomers of the same organic molecule
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
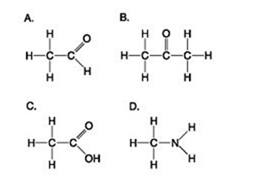 Which molecule has at least one carbon atom attached to three different chemical groups?
Which molecule has at least one carbon atom attached to three different chemical groups?
A) A
B) B
C) D
D) A and B
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 58 of 58
Related Exams