A) carbonyl and amino groups
B) carboxyl and amino groups
C) amino and sulfhydryl groups
D) hydroxyl and carboxyl groups
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an ethane (C₂H₆) molecule, each carbon atom is bonded to ________ hydrogen atoms.
A) two
B) three
C) four
D) six
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The kind and number of bonds an atom can form depends on ________.
A) its atomic number
B) its electron configuration
C) its atomic mass
D) the number of particles in its nucleus
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figure to answer the question.
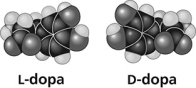 Thalidomide and L-dopa (see figure) are examples of pharmaceutical drugs that occur as enantiomers, or molecules that ________.
Thalidomide and L-dopa (see figure) are examples of pharmaceutical drugs that occur as enantiomers, or molecules that ________.
A) have identical three-dimensional shapes
B) are mirror images of one another
C) are mirror images of one another and have the same biological activity
D) are cis-trans isomers
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration?
A) the presence or absence of bonds with oxygen atoms
B) the presence or absence of double bonds between the carbon atom and other atoms
C) the polarity of the covalent bonds between carbon and other atoms
D) the solvent in which the organic molecule is dissolved
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
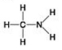 B.
B.
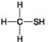 C.
C.
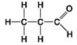 D.
D.
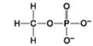 Which molecule shown above contains a functional group that is a part of the molecule known as the "energy currency of living organisms"?
Which molecule shown above contains a functional group that is a part of the molecule known as the "energy currency of living organisms"?
A) A
B) B
C) C
D) D
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Each bond in carbon dioxide represents ________.
O  C
C  O
O
A) one resonating electron
B) a pair of shared electrons
C) two pairs of shared electrons
D) a pair of protons
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
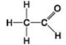 B.
B.
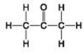 C.
C.
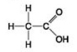 D.
D.
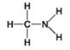 Which molecule shown has a carbonyl functional group in the form of an aldehyde?
Which molecule shown has a carbonyl functional group in the form of an aldehyde?
A) A
B) B
C) C
D) D
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Visualize the structural formula of each of the following hydrocarbons. Which hydrocarbon has a double bond in its carbon skeleton?
A) C₃H₈
B) C₂H₆
C) C₂H₄
D) C₂H₂
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Amino acids are acids because they always possess ________ as the functional group?
A) amino
B) carbonyl
C) carboxyl
D) phosphate
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which action could produce a carbonyl group?
A) the replacement of the -OH of a carboxyl group with hydrogen
B) the addition of a thiol to a hydroxyl
C) the addition of a hydroxyl to a phosphate
D) the replacement of the nitrogen of an amine with oxygen
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the term that correctly describes the relationship between these two sugar molecules:
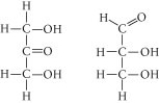
A) structural isomers
B) cis-trans isomers
C) enantiomers
D) isotopes
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
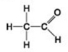 B.
B.
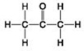 C.
C.
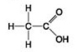 D.
D.
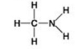 Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
A) A
B) B
C) C
D) D
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
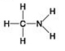 B.
B.
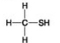 C.
C.
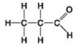 D.
D.
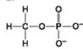 Which molecule can be a result of mercaptoethanol reduction of a disulfide bridge?
Which molecule can be a result of mercaptoethanol reduction of a disulfide bridge?
A) A
B) B
C) C
D) D
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Stanley Miller's 1953 experiments supported the hypothesis that ________.
A) life on Earth arose from simple inorganic molecules
B) organic molecules can be synthesized abiotically under conditions that may have existed on early Earth
C) life on Earth arose from simple organic molecules, with energy from lightning and volcanoes
D) the conditions on early Earth were conducive to the origin of life
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complexity and variety of organic molecules is due to ________.
A) the chemical versatility of carbon atoms
B) the variety of rare elements in organic molecules
C) the diverse bonding patterns of nitrogen
D) their interaction with water
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
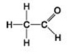 B.
B.
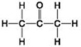 C.
C.
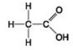 D.
D.
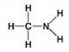 Which molecule shown contains a carboxyl group?
Which molecule shown contains a carboxyl group?
A) A
B) B
C) C
D) D
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
A.
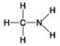 B.
B.
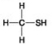 C.
C.
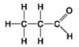 D.
D.
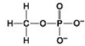 Which molecule shown above can contribute negative charge when positioned in a chain?
Which molecule shown above can contribute negative charge when positioned in a chain?
A) A
B) B
C) C
D) D
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question.
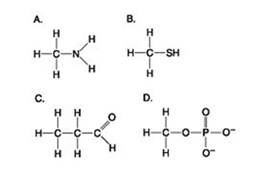 Which molecules shown contain a carbonyl group?
Which molecules shown contain a carbonyl group?
A) A and B
B) B and C
C) B, C, and D
D) C and D
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
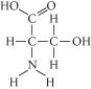 Which functional group is not present in this molecule?
Which functional group is not present in this molecule?
A) carboxyl
B) sulfhydryl
C) hydroxyl
D) amino
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 58
Related Exams