A) 0
B) +1
C) +2
D) +3
E) +4
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s) . What does the symbol represent?
A) Electron configuration
B) Valence electrons
C) Atomic number
D) Atomic mass
E) Nucleus and core electrons
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is wrong with this Lewis structure? 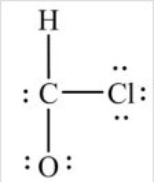
A) There are too many electrons.
B) There are too few electrons.
C) The O atom does not have an octet.
D) The C atom does not have an octet.
E) There is nothing wrong with this Lewis structure.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on ionic radii and charges, which of the following solids would have the greatest lattice energy?
A) SrO
B) CsI
C) MgCl2
D) KF
E) NaF
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
When an alkali metal combines with a nonmetal, a covalent bond is normally formed.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many single bonds are bonded to sulfur in SF6?
A) 2
B) 4
C) 6
D) 7
E) 8
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Describe, with appropriate explanations, the key factors which affect the magnitude of the lattice energy of an ionic substance.
Correct Answer

verified
By Coulomb's law, the energy of two elec...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The total number of bonding electrons in a molecule of formaldehyde (H2CO) is
A) 3.
B) 4.
C) 6.
D) 8.
E) 18.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the correct equation to calculate the formal charge on an atom.
A) formal charge = valence electrons - lone pair electrons
B) formal charge = valence electrons - associated electrons
C) formal charge = valence electrons - bonding electrons
D) formal charge = bonding electrons + lone pair electrons
E) formal charge = valence electrons - bonding electrons + lone pair electrons
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a covalent compound?
A) Na2O
B) CaCl2
C) Cl2O
D) CsCl
E) Al2O3
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The number of dots in the Lewis dot symbol for a main group element is the same as the ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis dot symbol consists of the symbol for the element surrounded by dot(s) . What does the symbol represent?
A) Electron configuration
B) Valence electrons
C) Atomic number
D) Atomic mass
E) Nucleus and core electrons
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which response includes all the molecules below that have a central atom that does not follow the octet rule? (1) H2S, (2) BCl3, (3) PH3, (4) SF4
A) (2) and (4)
B) (2) and (3)
C) (1) and (2)
D) (3) and (4)
E) (1) and (4)
G) C) and D)
Correct Answer

verified
A
Correct Answer
verified
Short Answer
________ of atoms interact to form compounds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the best Lewis structure for the fulminate ion, CNO-, what is the formal charge on the central nitrogen atom?
A) +2
B) +1
C) 0
D) -1
E) -2
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many dots does the Lewis dot symbol for argon have around it?
A) 1
B) 2
C) 4
D) 10
E) 8
G) A) and B)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Which of the following represents the most polar bond?
A) B-C
B) S-O
C) C-O
D) B-O
E) C-C
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the element whose Lewis symbol is correct.
A) •Ga•
B) ![]()
C) Al•
D) ![]()
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis dot symbol for the S2- ion is
A) ![]()
B) ![]()
C) S2-
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these substances will display an incomplete octet in its Lewis structure?
A) CO2
B) Cl2
C) ICl
D) NO
E) SO2
G) A) and C)
Correct Answer

verified
D
Correct Answer
verified
Showing 1 - 20 of 102
Related Exams