B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Liquid sodium can be used as a heat transfer fluid. Its vapor pressure is 40.0 torr at 633°C and 400.0 torr at 823°C. Calculate its heat of vaporization.
A) 43.4 kJ/mol
B) 52.5 kJ/mol
C) 70.6 kJ/mol
D) 1.00 × 10 2 kJ/mol
E) None of these choices are correct.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
If the solid form of a pure substance is denser than its liquid form, an increase in pressure will cause the melting point to decrease.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The energy of a hydrogen bond is greater than that of a typical covalent bond.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the solid forms of the following elements, which one is most likely to be of the molecular type?
A) Xe
B) C
C) Pb
D) S
E) Cr
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When liquid bromine is cooled to form a solid, which of the following types of solid would it form?
A) atomic
B) metallic
C) molecular
D) ionic
E) covalent network
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
All gases can be liquefied at room temperature simply by increasing the pressure on the gas.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following should have the highest boiling point?
A) CF 4
B) CCl 4
C) CBr 4
D) CI 4
E) CH 4
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The strongest intermolecular interactions between ethyl alcohol (CH3CH2OH) molecules arise from
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) carbon-oxygen bonds.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The strongest intermolecular interactions between hydrogen sulfide (H2S) molecules arise from
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) ion-dipole interactions.
E) disulfide linkages.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Polonium crystallizes in the simple cubic lattice. What is the coordination number for Po?
A) 3
B) 4
C) 6
D) 8
E) 12
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ammonia's unusually high melting point is the result of
A) dipole-dipole forces.
B) London dispersion forces.
C) hydrogen bonding.
D) covalent bonding.
E) ionic bonding.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What adjective best describes the solid compound IF7?
A) metallic
B) amorphous
C) covalent network
D) molecular
E) ionic
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following statements about unit cells and packing in solids is incorrect?
A) In any unit cell of a solid crystal, each face of the cell must have an opposite face which is equal and parallel to it.
B) The faces of a unit cell must all be at angles of 90° to each other.
C) The coordination number of atoms in a close packed metal is 12.
D) The packing efficiency in fcc structures is higher than in bcc structures.
E) The packing efficiency in fcc and hcp structures is the same.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Liquid ammonia (boiling point = −33.4°C) can be used as a refrigerant and heat transfer fluid. How much energy is needed to heat 25.0 g of NH3(l) from −65.0°C to −12.0°C? 
A) 5.5 kJ
B) 6.3 kJ
C) 39 kJ
D) 340 kJ
E) 590 kJ
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Examine the following phase diagram and determine what phase exists at point F. 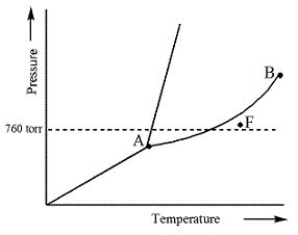
A) Vapor + Liquid
B) Vapor
C) Liquid
D) Solid
E) Supercritical fluid
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The normal boiling point of ether is 307.8 K. Calculate the temperature at which its vapor pressure is exactly half of that at its normal boiling point. The heat of vaporization for ether is 26.69 kJ/mol.
A) 305 K
B) 302 K
C) 295 K
D) 289 K
E) 281 K
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following factors contributes to a low viscosity for a liquid?
A) Low temperature
B) Spherical molecular shape
C) Hydrogen bonding
D) High molecular weight
E) High boiling point
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the pair of substances in which the one with the lower vapor pressure at a given temperature is listed first.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When identical particles pack in a simple cubic lattice, there is/are __________ particle(s) per unit cell.
A) 1
B) 2
C) 3
D) 4
E) 8
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 95
Related Exams