A) atoms
B) moles of atoms
C) moles of molecules
D) mass
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equations is balanced?
A) P4(s) + 10O2(g) P4O10(s)
B) ZnS(s) + 3O2(g) ZnO(s) + 2SO2(g)
C) NH3(g) + O2(g) NO2(g) + H2O(g)
D) 4KBrO3(s) 3KBrO4(s) + KBr(s)
E) 2Na(s) + P(s) Na3P(s)
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When mixed, solutions of silver nitrate, AgNO3, and sodium phosphate, Na3PO4, will form a precipitate of silver phosphate, Ag3PO4.The balanced equation is 3AgNO3(aq) + Na3PO4(aq) Ag3PO4(s) + 3NaNO3(aq) Which of the following statements regarding this reaction is incorrect?
A) 6 moles of AgNO3 will react with 2 moles of Na3PO4.
B) 9 moles of AgNO3 should react to form 2 moles of Ag3PO4, given sufficient Na3PO4.
C) 1.5 moles of NaNO3 should form when 0.5 mole of Na3PO4 reacts with sufficient AgNO3.
D) 3 moles of Ag3PO4 should form when 3 moles of Na3PO4 react with sufficient AgNO3.
E) 2 moles of Na3PO4 will react with 6 moles of AgNO3.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When ethanol, C2H5OH, a component in some gasoline mixtures, is burned in air, one molecule of ethanol combines with three oxygen molecules to form two CO2 molecules and three H2O molecules.Select the statement below that is incorrect in regard to this reaction.
A) The balanced equation for the reaction is C2H5OH + 3O2 2CO2 + 3H2O.
B) If 4 ethanol molecules react, 8 CO2 molecules should form.
C) If 12 H2O molecules are formed, then 9 O2 molecules must have reacted.
D) If 15 ethanol molecules react, then 45 oxygen molecules must also react.
E) If 12 CO2 molecules are formed, then 18 H2O molecules should also form.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When copper reacts with sulfur at high temperature, copper(I) sulfide is formed. 2Cu(s) + S(s) Cu2S(s) If the mass of the Cu2S formed is 1.17 g, what mass of copper should have reacted?
A) 0.934 g
B) not enough information
C) 0.78 g
D) 2.34 g
E) 0.467 g
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the following equation: ___P4(s) + ___Cl2(g) ___PCl3(l) (unbalanced) Balance the equation and determine how many grams of chlorine would be required to react with 21.2 g of phosphorus.
A) 12.1 g
B) 48.6 g
C) 37.0 g
D) 148 g
E) 72.8 g
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When magnesium is heated in air, it reacts with oxygen to form magnesium oxide: 2Mg(s) + O2(g) 2MgO(s) If the mass of the magnesium solid increases by 0.335 g, what mass of magnesium metal should have reacted?
A) 0.882 g
B) 0.441 g
C) 0.509 g
D) 1.02 g
E) not enough information
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction: Cr2O3(s) + 3CCl4(l) 2CrCl3(s) + 3COCl2(g) green colorless purple colorless solid liquid solid gas When the green solid is mixed with the colorless liquid, the mixture starts to bubble and fume.When all action has stopped, a wet purple solid remains.Which substance is the limiting reactant?
A) Cr2O3
B) CCl4
C) CrCl3
D) COCl2
E) there is no limiting reactant
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When mixed, solutions of copper(II) nitrate, Cu(NO3) 2, and sodium phosphate, Na3PO4, will form a precipitate of copper phosphate, Cu3(PO4) 2.The balanced equation is 3Cu(NO3) 2(aq) + 2Na3PO4(aq) Cu3(PO4) 2(s) + 6NaNO3(aq) Which of the following statements regarding this reaction is incorrect?
A) 8 moles of Na3PO4 will react with 12 moles of Cu(NO3) 2.
B) 1 mole of NaNO3 should form when 0.5 mole of Cu(NO3) 2 reacts with sufficient Na3PO4.
C) 12 moles of Cu3(PO4) 2 should form when 36 moles of Cu(NO3) 2 reacts with sufficient Na3PO4.
D) If 10 moles of Na3PO4 react with sufficient Cu(NO3) 2, 4 moles of Cu3(PO4) 2 should form.
E) If 5 moles of Cu3(PO4) 2 are needed, it would require the combination of 15 moles of Cu(NO3) 2 and 10 moles of Na3PO4.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 3.50 g sample of rice was burned in a bomb calorimeter containing 1980 g of water.The temperature of the water increased from 22.75 C to 28.88 C.How much heat, in joules, did the rice sample release when it burned? [Cwater = 4.184 J/(g C) ]
A) 1.88 x 105 J
B) 2.39 x 105 J
C) 5.08 x 104 J
D) 3.33 x 102 J
E) 4.22 x 102 J
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When one molecule of propane, C3H8, burns in a gas grill, it combines with five oxygen molecules to form three CO2 molecules and four H2O molecules.Select the statement below that is incorrect in regard to this reaction.
A) The balanced equation for the reaction is C3H8 + 5O2 3CO2 + 4H2O.
B) If 5 propane molecules react, 15 CO2 molecules should form.
C) If 5 propane molecules react, 25 O2 molecules must also react.
D) If 15 O2 molecules react, 9 H2O molecules should form.
E) If 12 CO2 molecules are formed, then 4 propane molecules must have reacted.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iron metal reacts with hydrochloric acid as follows: 2Fe(s) + 6HCl(aq) 2FeCl3(aq) + 3H2(g) If 22.4 g of iron react with excess HCl, and 59.4 g of FeCl3 are collected, what is the percent yield of Al2(SO4) 3?
A) 65.0%
B) 109%
C) 91.4%
D) 73.0%
E) not enough information given
G) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Small amounts of oxygen gas can be produced for laboratory use by heating potassium chlorate, which causes it to decompose by the following reaction: ___KClO3(s) ____KCl(s) + ___O2(g) (unbalanced) Balance the equation, and determine the mass of oxygen that should be formed if 15.0 g of potassium chlorate decomposes.
A) 11.7 g
B) 57.5 g
C) 173 g
D) 5.88 g
E) 86.1 g
G) B) and D)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Consider the reaction between acetylene, C2H2, and oxygen in a welding torch: 2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(g) Which of the following is not conserved in this reaction?
A) atoms
B) moles of atoms
C) moles of molecules
D) mass
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) , if 4.5 moles of NH3 react with sufficient oxygen, how many moles of H2O should form?
A) 4.0
B) 4.5
C) 6.0
D) 6.8
E) 5.5
G) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
The limiting reactant in a chemical reaction is always the reactant which is present in the least amount in terms of mass.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
In a bomb calorimeter, the bomb itself, the water surrounding it, and the thermometer would be considered part of the "system."
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 5.0 g CaCl2 is dissolved in water, how many moles of ions are in solution?
A) 0.135 mole
B) 0.045 mole
C) 5.0 moles
D) 15.0 moles
E) 1660 moles
G) D) and E)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Consider the reaction N2(g) + O2(g) 2NO(g) .The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs.What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 2 N2 molecules and 4 O2 molecules. 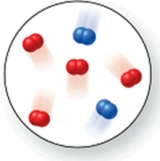
A) N2(g) , 1 O2(g)
B) N2(g) , 2 O2(g)
C) N2(g) , 3 O2(g)
D) O2(g) , 1 N2(g)
E) N2(g) , 0 O2(g)
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When phosphorus reacts with chlorine, phosphorus trichloride is formed according to the equation: ___P4(s) + ___Cl2(g) ___PCl3(l) (unbalanced) Balance the equation and determine how many grams of chlorine would be required to react with 10.6 g of phosphorus.
A) 6.07 g
B) 24.3 g
C) 36.4 g
D) 18.5 g
E) 74.1 g
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 120
Related Exams