A) zero joules
B) 20 J
C) 40 J
D) 1000 J
E) 1340 J
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One mole of a monatomic gas at 433 K is reversibly taken to half of its original volume by an isobaric process.How much work is done by the gas?
A) +1800 J
B) -1800 J
C) +3600 J
D) -3600 J
E) -8400 J
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
15-4 Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi.The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi. -What is the change in entropy of the sample?
A) +8.31 (ln 2) J/K
B) -8.31 (ln 2) J/K
C) +16.62 (ln 2) J/K
D) -16.62 (ln 2) J/K
E) zero J/K
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A container is divided into two chambers that are separated by a valve.The left chamber contains one mole of a monatomic ideal gas.The right chamber is evacuated.At some instant, the valve is opened and the gas rushes freely into the right chamber.Which one of the following statements concerning this process is true? 
A) Work is done by the gas.
B) The temperature of the gas decreases.
C) The change in the entropy of the gas is zero.
D) The walls of the containing vessel must get colder.
E) The change in the internal energy of the gas is zero.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete the following statement: Walls that separate a system from its surroundings and permit heat to flow through them are called
A) diathermal walls.
B) adiabatic walls.
C) entropic walls.
D) isobaric walls.
E) isochoric walls.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
15-1
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.  -Neon is a monatomic gas with a molar heat capacity at constant volume of 12.66 J/(mol · K) .Two moles of neon gas enclosed in a constant volume system receive 4250 J of heat.If the gas was initially at 293 K, what is the final temperature of the neon?
-Neon is a monatomic gas with a molar heat capacity at constant volume of 12.66 J/(mol · K) .Two moles of neon gas enclosed in a constant volume system receive 4250 J of heat.If the gas was initially at 293 K, what is the final temperature of the neon?
A) 3400 K
B) 400 K
C) 460 K
D) 520 K
E) 600 K
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ideal monatomic gas undergoes an adiabatic process; and its internal energy decreases by 50 J.Which pair of choices below is correct for this process? work done heat exchanged
A) 50 J by the system zero joules
B) 50 J on the system zero joules
C) 50 J by the system 100 J supplied
D) zero joules 50 J removed
E) zero joules 50 J added
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
15-3
An ideal monatomic gas expands isobarically from state A to state B.It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.  -How much work is done by the gas in expanding isobarically from A to B?
-How much work is done by the gas in expanding isobarically from A to B?
A) 1 × 103 J
B) 2 × 103 J
C) 3 × 103 J
D) 4 × 103 J
E) 5 × 103 J
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A paddle wheel frictionally adds thermal energy to 4.0 moles of an ideal monatomic gas in a sealed insulated container.The paddle wheel is driven by a cord connected to a falling object as shown in the drawing.How far has the 5.0-kg object fallen when the temperature of the gas increases by 7.50 K? 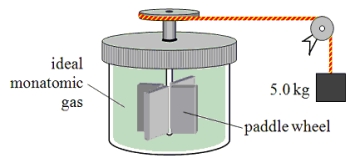
A) 7.6 m
B) 9.1 m
C) 11 m
D) 13 m
E) 15 m
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fixed amount of ideal gas is compressed adiabatically.Which entry in the table below correctly indicates the sign of the work done, the change in the internal energy, and the heat exchanged with the environment? work done change in internal energy heat exchanged
A) positive negative zero
B) negative zero positive
C) negative negative zero
D) positive positive zero
E) negative positive zero
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
15-2
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph. 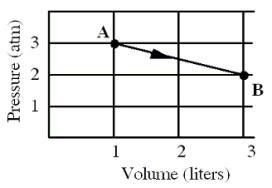 -What is the change in the internal energy, in calories, of the gas for this process?
-What is the change in the internal energy, in calories, of the gas for this process?
A) zero calories
B) +12 cal
C) -110 cal
D) +110 cal
E) +122 cal
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
15-6 A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer. -How much heat is extracted from the water in this process?
A) 48 000 J
B) 170 000 J
C) 400 000 J
D) 550 000 J
E) 348 000 J
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an isothermal process, 1.59 moles of an ideal gas is compressed to one-fifth of its initial volume at 285 K.What quantity of heat is added to, or removed from, the system during this process?
A) 133 J added
B) 3020 J added
C) 1070 J removed
D) 6060 J removed
E) 8880 J removed
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A match ignites within in an oxygen-filled cylinder that has a movable piston.The piston is moved so quickly that no heat escapes.What kind of change is demonstrated in this process?
A) an isobaric change
B) an adiabatic change
C) an isothermal change
D) an isochoric change
E) a change of heat capacity
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
15-1
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.  -Heat is added to a sample of an ideal monatomic gas.Which one of the following statements is necessarily true?
-Heat is added to a sample of an ideal monatomic gas.Which one of the following statements is necessarily true?
A) The gas must expand.
B) The gas must do work.
C) The type of change that will occur depends on the conditions of the gas when the heat was added.
D) The gas must change phase.
E) The temperature of the gas must increase.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
15-6 A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer. -Which one of the following processes represents a decrease in entropy?
A) the melting of ice
B) the sublimation of carbon dioxide
C) the evaporation of perfume
D) the vaporization of liquid helium
E) the condensation of steam on a kitchen window
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
15-6 A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer. -In which one of these processes will there be no net change in the entropy of the system?
A) the growth of a microorganism
B) the fusion of a crystalline solid
C) the heating of water in an open container
D) the combustion of fuel in a machine engine
E) the evaporation and condensation of benzene in a closed vessel
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
During one stage of a reversible process, the temperature of an ideal gas remains constant as its volume is decreased.Which one of the following statements concerning this situation is true?
A) The process is adiabatic.
B) The pressure of the gas decreases in this process.
C) Heat flows out of the gas and into the surroundings.
D) The gas does "positive" work on its surroundings.
E) The average kinetic energy of the gas molecules increases.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following statements is true concerning the ratio of the molar heat capacities Cp/Cv for an ideal gas?
A) The ratio is always 1.
B) The ratio is always less than 1.
C) The ratio is always greater than 1.
D) The ratio is sometimes less than 1.
E) The ratio is sometimes greater than 1.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A Carnot engine operates between hot and cold reservoirs with temperatures 527 °C and -73.0 °C, respectively.If the engine performs 1000.0 J of work per cycle, how much heat is extracted per cycle from the hot reservoir?
A) 878 J
B) 1333 J
C) 1163 J
D) 1527 J
E) 2010 J
G) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 79
Related Exams