A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Fill in missing sub- and superscripts for all particles to complete the following equation for beta decay. 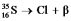
Correct Answer

verified
Correct Answer
verified
True/False
Positron decay and electron capture have the same net effect on the Z and N values of a nucleus.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radioisotope 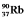 will decay through
will decay through
A) ( decay.)
B) ( decay.)
C) positron decay.
D) electron capture.
E) ( decay.)
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Gamma rays are not deflected by an electric field.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Write a complete, balanced equation to represent the formation of manganese-55 by the beta decay of another nuclide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a subatomic particle closely related to the positron?
A) proton
B) electron
C) negatron
D) neutron
E) neutrino
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Write a complete, balanced equation to represent the beta decay of thallium-207.
Correct Answer

verified
Correct Answer
verified
True/False
No alpha decay is observed for isotopes of elements with Z < 83.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 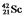 is unstable. This is predictable because
is unstable. This is predictable because
A) the number of neutrons is too large in relation to the number of protons.
B) the number of neutrons is too small in relation to the number of protons.
C) the atomic number is too large.
D) the mass number is too large.
E) Sc isotopes are all unstable.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radioisotope 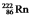 will decay through
will decay through
A) ( decay.)
B) ( decay.)
C) ( decay.)
D) positron decay.
E) electron capture.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 7.85 *10¯5 mol sample of copper-61 emits 1.47 *1019 positrons in 90.0 minutes. What is the decay constant for copper-61?
A) 0.00230 h¯1
B) 0.00346 h¯1
C) 0.207 h¯1
D) 0.311 h¯1
E) None of these choices is correct.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radiochemist, Will I. Glow, studied thorium-232 and found that 2.82 *10¯7 moles emitted 8.42 *106 particles in one year. What is the decay constant for thorium-232?
A) 3.35 * 10¯14 yr¯1
B) 4.96 *10¯11 yr¯1
C) 1.40 * 1010 yr¯1
D) 2.99 * 1013 yr¯1
E) None of these choices is correct.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction: 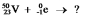
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices is correct.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing species in the following nuclear transmutation: 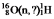
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction: 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotopes 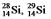 and
and 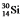 are all stable, while
are all stable, while 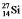 is radioactive. The mode of decay for
is radioactive. The mode of decay for 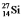 is most likely to be
is most likely to be
A) positron decay.
B) alpha decay.
C) beta decay.
D) gamma decay.
E) fission.
G) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
The (negative) binding energy per nucleon reaches a maximum for the isotope 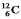 .
.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is an incorrect representation of the indicated particle or nucleus?
A) positron: ![]()
B) neutron: ![]()
C) helium-3: ![]()
D) alpha particle: ![]()
E) proton: ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction: 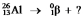
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 90
Related Exams