A) ( decay.)
B) ( decay.)
C) ( decay.)
D) electron capture.
E) spontaneous fission.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An isotope with a high value of N/Z will tend to decay through
A) ( decay.)
B) ( decay.)
C) positron decay.
D) electron capture.
E) ( decay.)
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction: 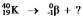
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calcium-39 undergoes positron decay. Each positron carries 5.49 MeV of energy. How much energy will be emitted when 0.0025 mol of calcium-39 decays?
A) 13.2 kJ
B) 1.32 * 104 kJ
C) 1.32 *106 kJ
D) 1.32 * 109 kJ
E) None of these choices is correct.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assuming that no other particles are produced, which of the following particles could be used to bombard nitrogen-14 in order to make fluorine-18?
A) alpha particle
B) beta particle
C) neutron
D) proton
E) positron
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Write a complete, balanced equation to represent the electron capture decay of argon-37.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following nuclei has a magic number of neutrons and/or protons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
What features do the r- and s-processes for element formation have in common? How do they differ?
Correct Answer

verified
Both involve neutron absorption by a nuc...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The isotopes 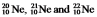 are all stable, while
are all stable, while 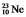 is radioactive. The mode of decay for
is radioactive. The mode of decay for 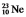 is most likely to be
is most likely to be
A) positron decay.
B) ( decay.)
C) ( decay.)
D) electron capture.
E) ( decay.)
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All the disintegrations of a sample of an unknown nuclide weighing 4.6 *10¯2 g were counted. In the first half-life of the sample, the total number of disintegrations counted was 4.3 *1020. What is the atomic weight of the unknown element?
A) 32 amu
B) 16 amu
C) 8 amu
D) 4 amu
E) None of these choices is correct.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A scintillation counter
A) measures the signal coming from an ionized gas.
B) measures light emissions from excited atoms.
C) depends on an avalanche of electrons generated as a particle moves through a tube of argon gas.
D) detects high energy radiation better than low energy radiation.
E) detects an electric current in a gas.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following series of radioactive decays would convert Pa-234 to Ra-226?
A) beta, alpha, beta
B) alpha, alpha
C) beta, alpha, alpha, beta
D) beta, alpha, alpha
E) alpha, beta, gamma
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cesium-134 is a emitter with a half-life of 2.0 years. How much of a 2.50-g sample of cesium-134 will remain after 10 years?
A) 0.0024 g
B) 0.078 g
C) 0.25 g
D) 0.50 g
E) None of these choices is correct.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An 85-kg person exposed to barium-141 receives 2.5 * 105 particles, each with an energy of 5.2 *10¯13 J. How many rads does the person receive?
A) 2.4 *10¯20
B) 1.5 * 10¯7
C) 1.8 * 10¯16
D) 6.1 *10¯15
E) None of these choices is correct.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
After 4 half-lives, the fraction of a radioactive isotope which still remains is approximately one-eighth.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following descriptions relating to nuclear reactions is correct?
A) The ratio of neutrons to protons remains constant.
B) The number of protons plus neutrons remains constant.
C) The number of electron remains constant.
D) The total charge changes.
E) The total number of nucleons changes.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The r-process occurs during supernova explosions.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Palladium-107 undergoes decay (t1/2 = 6.5 *105 yr) to form silver-107. How long will it take for 0.150 mol of silver-107 to form from 1.25 mol of palladium-107?
A) 2.0 *107 y
B) 1.4 * 107 y
C) 1.2 * 106 y
D) 8.3 * 105 y
E) 1.2 * 105 y
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 90
Related Exams