Correct Answer

verified
Correct Answer
verified
True/False
The boiling point of a solution that contains 0.64 mol of Mg(NO3)2 in 1.00 kg of water is 100.98 °C.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Only solids dissolve in water to form solutions.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An unknown amount of water is added to 75 mL of a 3.5 M aqueous glucose solution. What can be said about the concentration of the resulting solution?
A) The concentration of the resultant glucose solution will be less than 3.5 M.
B) The concentration of the resultant glucose solution will be greater than 3.5 M.
C) The concentration of the resultant glucose solution will remain the same because the amount of glucose has not changed.
D) It is impossible to say anything about the concentration of the resultant glucose solution because the amount of added water has not been provided.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Magnesium hydroxide can be made by the reaction shown below. If a chemist requires 0.725 moles of NaOH for this reaction,what volume of a 1.50 M NaOH solution is needed to provide this amount? MgCl2(aq) + 2 NaOH(aq) → Mg(OH) 2(s) + 2 NaCl(aq)
A) 0.483 L of a 1.50 M NaOH solution
B) 0.967 L of a 1.50 M NaOH solution
C) 1.09 L of a 1.50 M NaOH solution
D) 967 mL of a 1.50 M NaOH solution
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of glucose (C6H12O6) are contained in 555 mL of a 1.77 M glucose solution?
A) 0.982 g C6H12O6
B) 177 g C6H12O6
C) 0.555 g C6H12O6
D) 0.177 g C6H12O6
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
The melting point of a solution prepared from 2.00 moles of NaCl in 1.00kg of water is ______ºC.
Correct Answer

verified
Correct Answer
verified
True/False
The solubility of KI in water at 20 °C is 140 g KI/100 g H2O. If 160 g of KI is mixed with 150 g of water,all of the KI will dissolve and the solution that results will be unsaturated.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of compounds will form a solution?
A) Benzene (C6H6) and hexane (C6H14)
B) Na2SO4 and benzene (C6H6)
C) NaCl and hexane (C6H14)
D) H2O and CCl4
E) More than one of the combinations above will form solutions.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which solution has the highest boiling point?
A) A solution formed by dissolving 0.75 mol of KCl in 1.00 kg of water.
B) A solution formed by dissolving 0.75 mol of glucose (C6H12O6) in 1.00 kg of water.
C) A solution formed by dissolving 0.75 mol of Ca(NO3) 2 in 1.00 kg of water.
D) A solution formed by dissolving 0.75 mol of Na3PO4 in 1.00 kg of water.
E) All of the solutions described have the same boiling point.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The presence of a solute reduces the vapor pressure of the solvent above the solution,raising its boiling point.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would increase the solubility of solid NaCl in water ________.
A) decreasing the temperature of the solution
B) increasing the temperature of the solution
C) decreasing the pressure of the solution
D) increasing the pressure of the solution
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Methanol (CH3OH)is soluble in water.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Ethylene glycol is more soluble in water than propane. 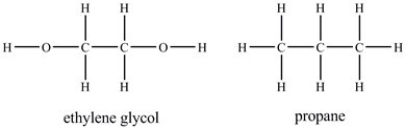
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is not an example of a solution?
A) A dental filling
B) Chicken noodle soup
C) Gasoline
D) Tap water
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The ionic compound CaCO3 is soluble in water.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A saline solution used in intravenous drips for patients who cannot take oral fluids contains 0.92% (w/v) NaCl in water. What volume of the saline solution must be administered to the patient in order to deliver 7.7 g of NaCl?
A) 8.4 mL of saline solution
B) 840 mL of saline solution
C) 7.1 mL of saline solution
D) 140 mL of saline solution
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Solubility is often summed up in three words: "________".
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The maximum level of lead allowed in drinking water is 15 mg/kg. What is this concentration in units of parts per million?
A) 15 ppm
B) 1.5 × 10-2 ppm
C) 1.5 × 104 ppm
D) 3.1 ppm
F) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
The solubility of Ba(NO3)2 in water is lower at 25 °C than at 50 °C.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 98
Related Exams