A) strongest covalent bond
B) longest covalent bond
C) weakest ionic bond
D) metallic bond
E) highest melting point
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many lone pairs of electrons are on the As atom in As Cl3?
A) 0
B) 1
C) 2
D) 3
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the best Lewis structure for BrO4⁻. What is the formal charge on bromine?
A) -1
B) +1
C) 0
D) +2
E) +3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of lone pairs for BF3.
A) 16
B) 8
C) 14
D) 10
E) 9
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the resonance structure that has the lowest formal charge on each atom for ClO2-. What is the formal charge on chlorine?
A) -1
B) +1
C) 0
D) +2
E) +3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the bond below that is the strongest.
A) C-F
B) C=O
C) C-I
D) I-I
E) C≡N
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for CO32-, including any valid resonance structures. Which of the following statements is TRUE?
A) The CO32- ion contains one C-O single bond and two C=O double bonds.
B) The CO32- ion contains three C-O bonds that are equivalent to 4/3 bonds.
C) The CO32- ion contains three C-O double bonds.
D) The CO32- ion contains two C-O single bonds and one C≡O triple bond.
E) None of the above is true.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A triple covalent bond contains ________ of electrons.
A) 0 pairs
B) 1 pair
C) 2 pairs
D) 3 pairs
E) 4 pairs
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for XeI2.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the resonance structure that has the lowest formal charge on each atom for BrO2-. What is the formal charge on bromine?
A) -1
B) +1
C) 0
D) +2
E) +3
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the bond below that is the weakest.
A) C≡O
B) N≡N
C) C-I
D) C=S
E) K-Cl
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the resonance structure that has the lowest formal charge on each atom for NO3-. What is the formal charge on nitrogen?
A) -1
B) +1
C) 0
D) +2
E) +3
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the data given below to construct a Born-Haber cycle to determine the bond energy of O2.
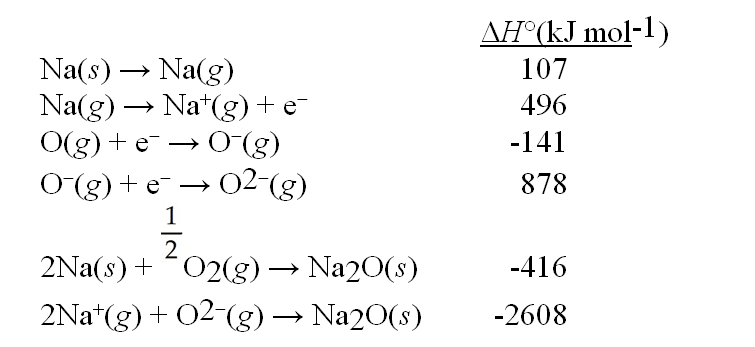
A) 426 kJ mol-1
B) 249 kJ mol-1
C) 852 kJ mol-1
D) 498 kJ mol-1
E) 356 kJ mol-1
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for ICl5.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following in order of decreasing magnitude of lattice energy. NaF RbBr KCl
A) RbBr > NaF > KCl
B) NaF > KCl > RbBr
C) KCl > NaF > RbBr
D) NaF > RbBr > KCl
E) RbBr > KCl > NaF
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements can form hypercoordinate compounds?
A) Se
B) C
C) Li
D) F
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use Lewis theory to determine the chemical formula for the compound formed between Al and O.
A) Al3O2
B) Al2O3
C) AlO2
D) Al2O
E) AlO
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is hypercoordinate?
A) CsI
B) SnO2
C) ClF5
D) ClF
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the longest bond.
A) single covalent bond
B) double covalent bond
C) triple covalent bond
D) All of the above bonds are the same length.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represent the Lewis structure for Mg?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 155
Related Exams