A) 32 amu
B) 16 amu
C) 8 amu
D) 4 amu
E) None of these choices are correct.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
No alpha decay is observed for isotopes of elements with Z < 83.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 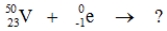
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An alkaline earth element is radioactive. It and its daughter elements decay by emitting a total of three alpha particles in succession. In what group of the periodic table is the element resulting from the emission of the third alpha particle?
A) 4A (14)
B) 5A (15)
C) 6A (16)
D) 7A (17)
E) 8A (18)
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotopes of promethium, 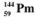 and
and 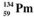 are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
A) promethium-144, β decay; promethium-134, positron decay
B) promethium-144, positron decay; promethium-134, β decay
C) promethium-144, positron decay; promethium-134, electron capture
D) promethium-144, electron capture; promethium-134, positron decay
E) promethium-144, β decay; promethium-134, ![]() decay
decay
G) A) and E)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which one of the following equations correctly represents alpha decay of 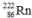 ?
?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
A
Correct Answer
verified
True/False
The r-process occurs during supernova explosions.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The s-process involves a slow succession of neutron absorption and beta decay processes during the normal life of a star.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following equations correctly represents positron decay of 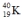 ?
?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 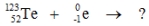
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain isotope has a specific activity of 7.29 × 10-4 Ci/g. How many α particles will a 75.0 mg sample emit in one hour?
A) 9.99 × 104
B) 2.02 × 106
C) 7.28 × 109
D) 1.29 × 1012
E) None of these choices are correct.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 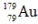 has a half-life of 7.5 seconds. If a sample contains 144 atoms of
has a half-life of 7.5 seconds. If a sample contains 144 atoms of 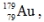 approximately how many such atoms were there present 30 seconds earlier?
approximately how many such atoms were there present 30 seconds earlier?
A) 576
B) 1152
C) 2304
D) 4320
E) 4.30 × 108
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Gamma rays are high energy electrons.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cesium-134 is a β emitter with a half-life of 2.0 years. How much of a 2.50-g sample of cesium-134 will remain after 10 years?
A) 0.0024 g
B) 0.078 g
C) 0.25 g
D) 0.50 g
E) None of these choices are correct.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
After 4 half-lives, the fraction of a radioactive isotope which still remains is approximately one-eighth.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 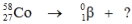
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radioisotope 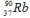 will decay through
will decay through
A) α decay.
B) β decay.
C) positron decay.
D) electron capture.
E) ![]() decay.
decay.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Most foodstuffs contain natural, radioactive isotopes.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 81
Related Exams