A) BaN
B) BaN2
C) Ba2N3
D) Ba2N
E) Ba3N2
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The more C-O and O-H bonds there are in a substance, the greater will be the amount of heat released when a fixed mass of the substance is burned.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct formula for a compound formed from calcium and chlorine.
A) CaCl
B) CaCl2
C) Ca2Cl
D) Ca2Cl2
E) CaCl3
G) C) and E)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Select the compound with the lowest (i.e., least negative) lattice energy.
A) CsBr(s)
B) NaCl(s)
C) SrO(s)
D) CaO(s)
E) KBr(s)
G) A) and B)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Select the element whose Lewis symbol is correct.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of the following elements (in their normal, stable, forms) would it be correct to describe the bonding as involving "electron pooling"?
A) hydrogen
B) helium
C) sulfur
D) iodine
E) aluminum
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen and hydrogen combine to form ammonia in the Haber process. Calculate (in kJ) the standard enthalpy change ΔH° for the reaction written below, using the bond energies given. N2(g) + 3H2(g) → 2NH3(g) Bond: N≡N H-H N-H Bond energy(kJ/mol) : 945 432 391
A) -969 kJ
B) -204 kJ
C) -105 kJ
D) 204 kJ
E) 595 kJ
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The lattice energy for ionic crystals increases as the charge on the ions _____________ and the size of the ions __________________.
A) increases; increases
B) increases; decreases
C) decreases; increases
D) decreases; decreases
E) None of these choices are correct.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following bonds in order of increasing bond strength.
A) C-I < C-Br < C-Cl < C-F
B) C-F < C-Cl < C-Br < C-I
C) C-Br < C-I < C-Cl < C-F
D) C-I < C-Br < C-F< C-Cl
E) None of these choices are correct.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following properties is least characteristic of typical ionic compounds?
A) high melting point
B) high boiling point
C) brittleness
D) poor electrical conductor when solid
E) poor electrical conductor when molten
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is the most electronegative?
A) Ne
B) Rb
C) P
D) I
E) Cl
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The melting points of metals are only moderately high because
A) metallic bonding is weak.
B) metals have fewer bonding electrons than nonmetals.
C) metals also have relatively low boiling points.
D) the melting process does not break the metallic bonds.
E) metals prefer to be bonded to nonmetals.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of these substances are the atoms held together by metallic bonding?
A) CO2
B) Si
C) Br2
D) S8
E) Cr
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following period 3 chlorides would be expected to have the highest melting point?
A) MgCl2
B) AlCl3
C) SiCl4
D) PCl3
E) SCl2
G) B) and E)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Calculate the lattice energy of magnesium sulfide from the data given below. Mg(s) → Mg(g) ΔH° = 148 kJ/mol Mg(g) → Mg2+(g) + 2e- ΔH° = 2186 kJ/mol S8(s) → 8S(g) ΔH° = 2232 kJ/mol S(g) + 2e- → S2-(g) ΔH° = 450 kJ/mol 8Mg(s) + S8(s) → 8MgS(s) ΔH° = -2744 kJ/mol Mg2+(g) + S2-(g) → MgS(s) ΔH°lattice = ?
A) -3406 kJ/mol
B) -2720. kJ/mol
C) 2720. kJ/mol
D) 3406 kJ/mol
E) None of these choices are correct.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
In covalent bond formation, the potential energy reaches a maximum when the internuclear distance is equal to the bond length.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The lattice energy of large ions is greater in magnitude than that of small ions of the same charge.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The majority of elements are good electrical conductors when in solid form.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon-oxygen double bond. Calculate the enthalpy of reaction using the bond energies given.
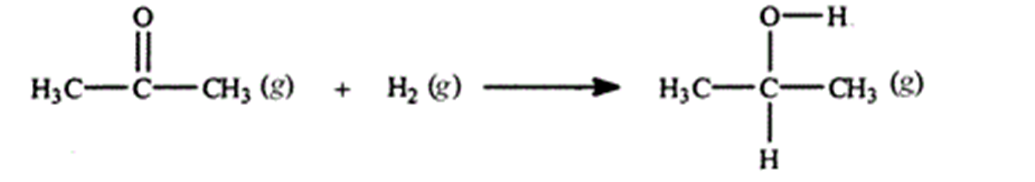
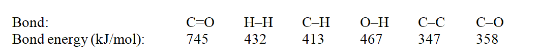
A) -484 kJ
B) -366 kJ
C) -61 kJ
D) +61 kJ
E) +366 kJ
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is the least electronegative?
A) Si
B) Se
C) S
D) Sc
E) Sr
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 61
Related Exams