A) The molecule must be large.
B) The molecule must contain a polar head.
C) The molecule must contain a non-polar tail.
D) The molecule must contain both a polar head and a non-polar tail.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is expected to be the least soluble in H2O? 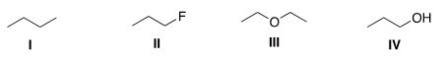
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkanes is expected to have the highest melting point? 
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of increasing strength of intermolecular forces. 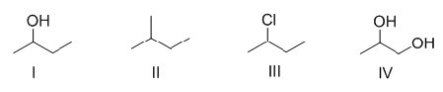
A) I > III > IV > II
B) IV > II > I > III
C) IV > I > III > II
D) I > IV > II > III
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules contain the same functional groups? 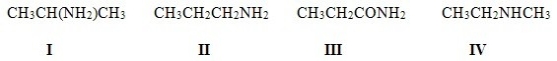
A) I,II,IV
B) I,II,III
C) II,III,IV
D) I,III,IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The indicated carbon atom is: 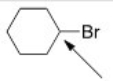
A) Electrophilic because it is electron-deficient.
B) Nucleophilic because it is electron-deficient.
C) Electrophilic because it is electron-rich.
D) Nucleophilic because it is electron-rich.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures contains an amide? 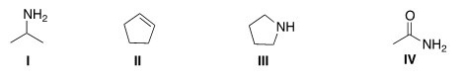
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest boiling point? 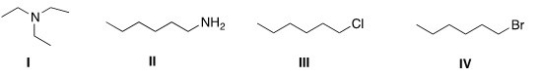
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of decreasing boiling point,putting the compound with the highest boiling point first. 
A) I > II > III > IV
B) III > IV > II > I
C) III > II > IV > I
D) I > IV > II > III
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly matches the molecules to the names of the functional group? I. CH3OH Carboxylic acid II) CH3CO2CH3 Ester III) CH3COCH3 Ketone IV) H2CO Alcohol
A) I and II
B) III and IV
C) II and III
D) II and IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures contains an alkene? 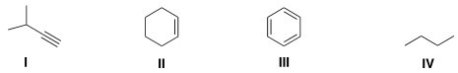
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following lists contains common heteroatoms found in organic molecules?
A) N,O,S,P,Cl
B) Na,O,S,P,Cl
C) Na,Mg,S,N,Cl
D) Na,Mg,O,N,Cl
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of decreasing melting point,putting the compound with the highest melting point first. 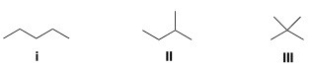
A) I > II > III
B) II > III > I
C) III > II > I
D) III > I > II
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What intermolecular force is generally considered the weakest?
A) Hydrogen bonding
B) London dispersion forces
C) Dipole-dipole
D) Ion-ion
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List the intermolecular forces present in the following molecule: 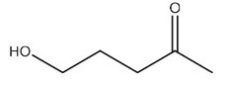
A) Van der Waals
B) Dipole-dipole interactions
C) Hydrogen bonding
D) More than one of these answer choices is correct.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements best describes the relationship between the surface area of a molecule and the strength of the intermolecular forces?
A) The larger the surface area,the weaker the intermolecular forces.
B) The larger the surface area,the stronger the intermolecular forces.
C) The smaller the surface area,the stronger the intermolecular forces.
D) There is no relationship between surface area and intermolecular forces.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 56 of 56
Related Exams