A) I
B) II
C) I and II
D) None of the choices
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the Aldol addition product formed from the reaction of (CH3) 2CHCH2CHO with itself? 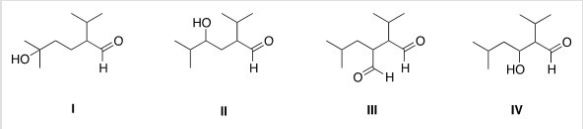
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What product (including stereochemistry) is formed in the following intermolecular reaction? 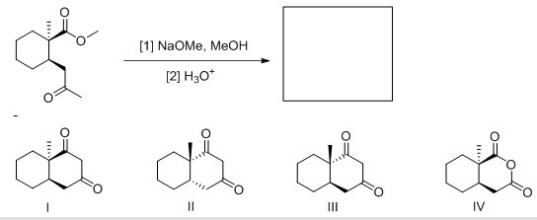
A) I
B) II
C) III
D) IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name given to an Aldol reaction between two different carbonyl compounds?
A) Multiple Aldol reaction
B) Crossed Aldol reaction
C) Differential Aldol reaction
D) Versatile Aldol reaction
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the Aldol addition product formed from reaction of the following compound with itself? 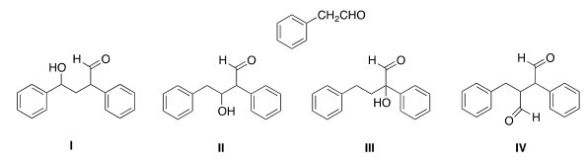
A) I
B) II
C) III
D) IV
F) None of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Complete this statement: A major difference between the Aldol condensation and the Claisen condensation reactions is that
A) the Aldol reaction involves substitution while the Claisen reaction involves addition.
B) the Aldol reaction is acid catalyzed while the Claisen reaction is base-catalyzed.
C) the Aldol reaction is base catalyzed while the Claisen reaction requires a full equivalent of base.
D) the Aldol reaction is base catalyzed while the Claisen reaction is acid-catalyzed.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What reaction type is an Aldol reaction?
A) Nucleophilic substitution
B) Electrophilic substitution
C) Electrophilic addition
D) Nucleophilic addition
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds cannot serve as a Michael acceptor? 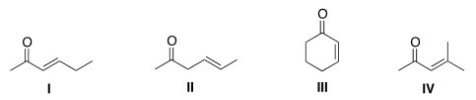
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In what situation can the yield of a single crossed Aldol product be increased?
A) The electrophilic carbonyl component is relatively unhindered and is used in excess.
B) The electrophilic carbonyl carbon component is relatively hindered and is used in limited amount.
C) The nucleophilic carbonyl component is relatively unhindered and is used in excess.
D) The nucleophilic carbonyl component is relatively hindered and is used in limited amount.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of esters can undergo Claisen reactions?
A) All esters can undergo Claisen reactions.
B) Only esters with two hydrogen atoms on the a carbon can undergo Claisen reactions.
C) Only esters with three hydrogen atoms on the a carbon can undergo Claisen reactions.
D) Only esters with two or three hydrogen atoms on the a carbon can undergo Claisen reactions.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the two starting materials for a Robinson annulation?
A) an a,b-Unsaturated carbonyl compound and an enolate
B) a b-Ketoester and an enolate
C) a 1,5-Dicarbonyl compound and an enolate
D) a 1,3-Dicarbonyl compound and an enolate
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When is a crossed Aldol reaction said to be synthetically useful?
A) When both carbonyl compounds have a hydrogens
B) When both carbonyl compounds have no a hydrogens
C) When one carbonyl compound has no a hydrogens
D) When one carbonyl compound has no b hydrogens
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following Claisen reaction? 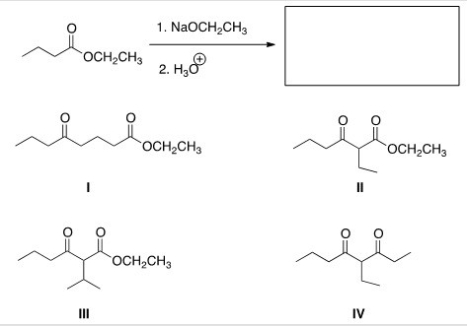
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
In a Michael reaction,where does the nucleophile attack the a,b-unsaturated carbonyl component?
A) a-Carbon
B) b-Carbon
C) Carbonyl carbon
D) Carbonyl carbon and b-carbon
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the correct order,what are the three steps in the mechanism of an Aldol reaction?
A) Protonation,enolate formation,nucleophilic addition
B) Enolate formation,protonation,nucleophilic addition
C) Enolate formation,nucleophilic addition,protonation
D) Nucleophilic addition,enolate formation,protonation
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Under basic conditions,the Aldol reaction is reversible,but dehydration is not.What is the reason for this difference in reactivity?
A) The initial Aldol product is an alkoxide,so the reaction is not energetically downhill in either direction.
B) The initial Aldol product is an alkoxide,so the reaction is energetically downhill going toward the product.
C) The initial Aldol product is an alkoxide,so the reaction is energetically downhill going toward the starting materials.
D) Water is a stable molecule.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction is an example of what type of reaction? 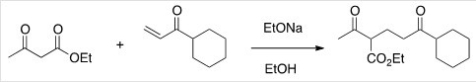
A) Michael reaction
B) Aldol self-condensation
C) Dieckmann condensation
D) Robinson annulation
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the cyclic product formed in the intramolecular Aldol condensation when the following compound is treated with aqueous NaOH? 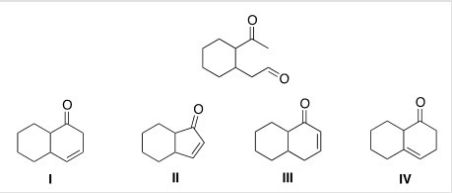
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The b-hydroxy carbonyl product of an Aldol reaction is oftentimes not the final isolated product; what is the explanation for this result?
A) It undergoes elimination,since water is a good leaving group.
B) The hydroxy group is oxidized to a carbonyl.
C) The hydroxy group reacts with the carbonyl to form a ketal.
D) Hydroxide is eliminated via an enolate intermediate.
F) A) and C)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
The following reaction is an example of what type of reaction? 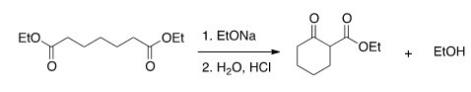
A) Claisen condensation
B) Mixed Aldol reaction
C) Robinson annulation
D) Dieckmann condensation
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 51
Related Exams