Correct Answer

verified
 cyclohexane,methylcyclopentan...
cyclohexane,methylcyclopentan...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Circle all bridgehead carbons in the following structure. 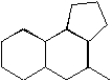
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 4-4
Label each pair of compounds below as:
-_____ 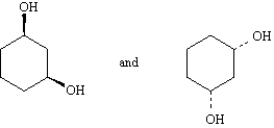
A) conformational isomers
B) stereoisomers
C) constitutional isomers
D) identical
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the two stereoisomers of 1,3-dibromocyclobutane.
Correct Answer

verified
Correct Answer
verified
Essay
The energy difference of 3.8 kJ/mol between gauche and anti butane corresponds to an equilibrium constant,Keq,of approximately 1.9.Calculate the percentage of each conformer at equilibrium.
Correct Answer

verified
Keq = (anti)/(gauche) = 1.9
(anti) = 1.9(g...View Answer
Show Answer
Correct Answer
verified
(anti) = 1.9(g...
View Answer
Essay
In general,5-alkyl substituents in 1,3-dioxane exhibit a smaller equatorial preference than they do in cyclohexane.To what might you attribute this observation? 
Correct Answer

verified
The 5-alkyl substituted dioxanes do not ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Name: 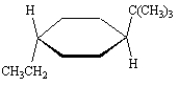
Correct Answer

verified
trans-1-tert-butyl-4...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw: cis-1-sec-butyl-2-ethylcyclopentane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 4-4
Label each pair of compounds below as:
-_____ 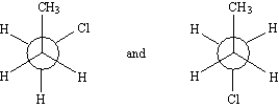
A) conformational isomers
B) stereoisomers
C) constitutional isomers
D) identical
F) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
D-Pinitol is an interesting hexahydroxy cyclohexane,whose structure is shown below. 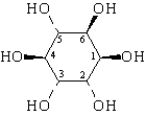 On the templates provided,draw the two chair conformations that are in equilibrium for D-pinitol.Circle the most stable conformation.
On the templates provided,draw the two chair conformations that are in equilibrium for D-pinitol.Circle the most stable conformation. 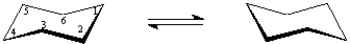
Correct Answer

verified
Correct Answer
verified
Essay
Exhibit 4-3
The following question(s) refer to the structure of camphor shown below. 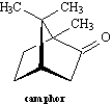 -Refer to Exhibit 4-3.Circle all bridgehead carbons in camphor.
-Refer to Exhibit 4-3.Circle all bridgehead carbons in camphor.
Correct Answer

verified
Correct Answer
verified
Essay
Exhibit 4-2
For each substituted cyclohexane below,draw its ring-flip isomer.Circle the most stable conformation.
-Refer to Exhibit 4-2. 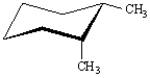
Correct Answer

verified
Correct Answer
verified
Essay
Draw: 1-chloro-2-isopropylcyclopentane
Correct Answer

verified
Correct Answer
verified
Short Answer
Exhibit 4-1
Refer to the structure below to answer the following question(s): 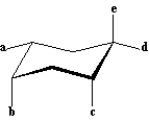 -Refer to Exhibit 4-1.Which of the labeled bonds in the structure are equatorial bonds?
-Refer to Exhibit 4-1.Which of the labeled bonds in the structure are equatorial bonds?
Correct Answer

verified
Correct Answer
verified
Essay
(-)-Menthol can be isolated from the peppermint plant and is responsible for the characteristic flavor and taste of peppermint.The structure of (-)-menthol is:  On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol.
On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following table. 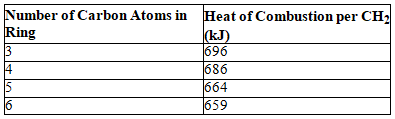 Based on the data in the table,which of the following compounds would have the largest strain energy?
Based on the data in the table,which of the following compounds would have the largest strain energy?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In cyclopropane,which of the following strain types would be the least important in determining the overall energy?
A) angle
B) torsional
C) steric
D) All make a significant contribution.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 37 of 37
Related Exams